- Language:English
- English
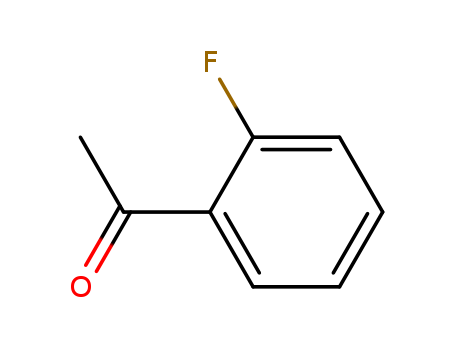

CasNo: 445-27-2
Molecular Formula: C8H7FO
Appearance: Clear colorless to light yellow or light green
|
Chemical Properties |
clear colorless to light yellow or light green |
|
Uses |
2'-Fluoroacetophenone is used as starting reagent in the synthesis of ascididemin. It is used to produce 1-(2-piperidin-1-yl-phenyl)-ethanone by reaction with piperidine. |
|
Synthesis Reference(s) |
Tetrahedron Letters, 31, p. 6179, 1990 DOI: 10.1016/S0040-4039(00)97018-7 |
|
General Description |
The enantioselective reduction of 2′-fluoroacetophenone has been investigated. |
Herein, we report a new protocol for the...
Conjugated microporous polymers (CMPs) h...
The Pd-cataylsed direct ortho-C(sp2)-H f...
With the increasing attention for green ...
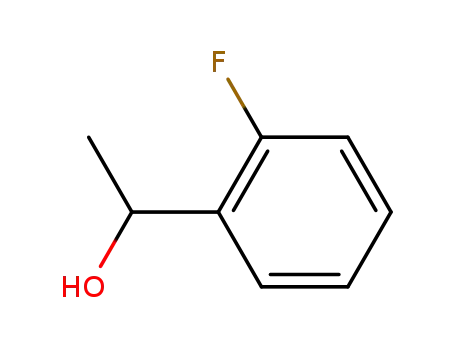
1-(2-fluorophenyl)ethanol

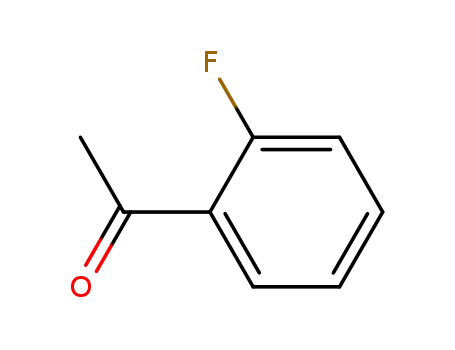
2'-Fluoroacetophenone
| Conditions | Yield |
|---|---|
|
With
[(2-(benzoimidazol-2-yl)-6-(3,5-dimethylpyrazol-1-yl)pyridine)RuCl2(PPh3)]; potassium tert-butylate; acetone;
In
methanol;
at 56 ℃;
for 0.5h;
under 750.075 Torr;
Catalytic behavior;
Inert atmosphere;
|
97% |
|
With
Langlois reagent;
In
acetonitrile;
at 25 ℃;
for 12h;
Irradiation;
Sealed tube;
|
89% |
|
With
calcomenite; potassium hydroxide;
In
toluene;
for 28h;
Reflux;
|
87% |
|
With
caesium carbonate;
In
toluene;
at 110 ℃;
for 18h;
|
77% |
|
With
tert.-butylhydroperoxide;
In
water;
at 100 ℃;
for 24h;
|
72% |
|
With
potassium tetrakis-μ-pyrophosphitodiplatinate(II);
In
water;
at 20 ℃;
for 8h;
Inert atmosphere;
Irradiation;
|
55% |
|
With
pyridinium chlorochromate;
In
dichloromethane;
|
|
|
With
sodium hypochlorite; N-Bromosuccinimide; [(R,R)-(N,N-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminato)]manganese(III) chloride;
In
dichloromethane; water;
at 25 ℃;
for 0.17h;
chemoselective reaction;
|
|
|
With
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; Trametes versicolor laccase;
In
tert-butyl methyl ether;
at 30 ℃;
for 16h;
pH=5;
Solvent;
Reagent/catalyst;
Catalytic behavior;
Enzymatic reaction;
|
|
|
With
[fac-N-(2-(diphenylphosphino)ethyl)-5,6,7,8-tetrahydroquinolin-8-amine]RuCl2(triphenylphosphine); potassium tert-butylate;
In
para-xylene;
for 24h;
Catalytic behavior;
Reflux;
Inert atmosphere;
|
|
|
With
oxygen;
at 120 ℃;
for 12h;
|
|
|
With
C44H52N2O4Ru; 4-methylmorpholine N-oxide;
In
acetonitrile;
at 82 ℃;
for 1h;
Inert atmosphere;
|
87 %Chromat. |
|
With
dipropylene glycol dimethyl ether; oxygen;
at 120 ℃;
|
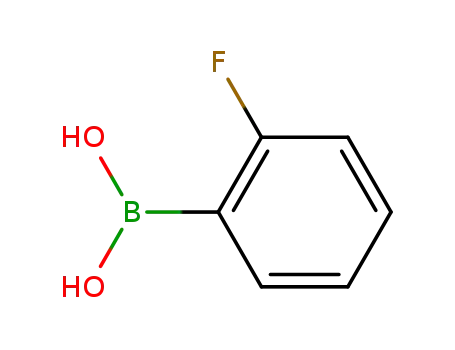
o-fluorophenylboronic acid


acetonitrile


2'-Fluoroacetophenone
| Conditions | Yield |
|---|---|
|
With
1,10-Phenanthroline; nickel(II) bromide diethylene glycol dimethyl ether; sodium hydrogencarbonate;
In
water;
at 100 ℃;
for 5h;
Autoclave;
|
89% |
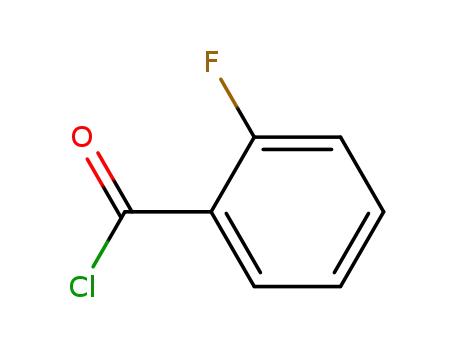
2-Fluorobenzoyl chloride

1-(2-fluorophenyl)ethanol
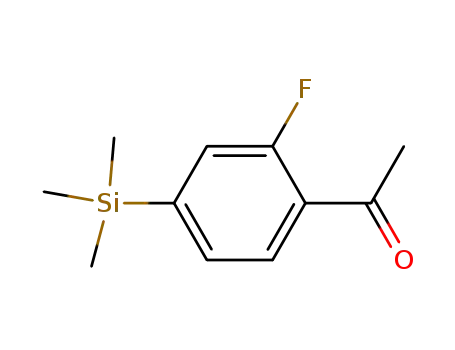
1-(2-Fluoro-4-trimethylsilanyl-phenyl)-ethanone
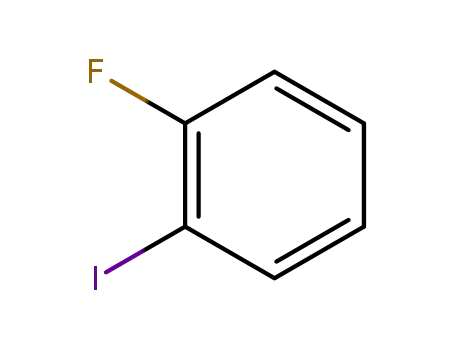
1-Fluoro-2-iodobenzene

1-fluoro-2-(1-phenylethenyl)benzene
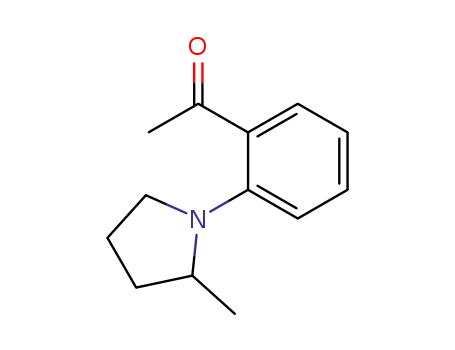
1-[2-(2-Methyl-pyrrolidin-1-yl)-phenyl]-ethanone
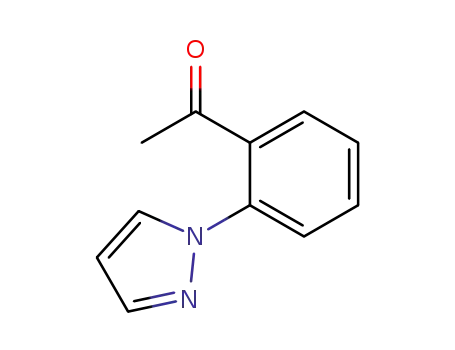
1-(2-(1H-pyrazol-1-yl)phenyl)ethan-1-one
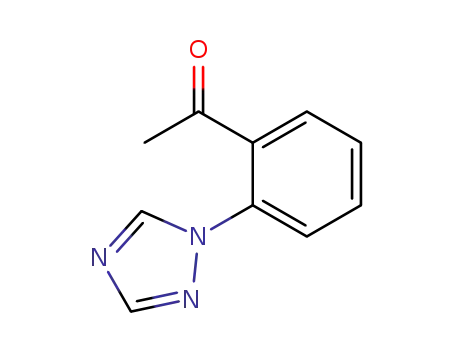
2'-(1,2,4-triazol-1-yl)acetophenone
CAS:99627-05-1
CAS:863329-66-2
CAS:1076-38-6
CAS:2142-68-9