- Language:English
- English
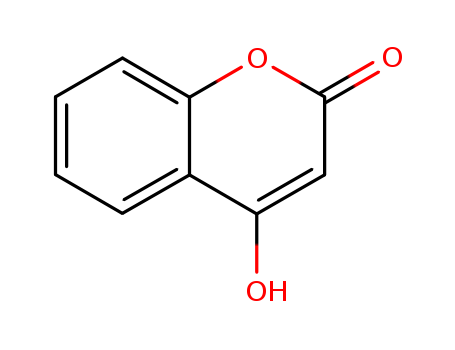

CasNo: 1076-38-6
Molecular Formula: C9H6O3
Appearance: Yellow powder or chunks
|
Chemical Properties |
Yellow powder or chunks or Slightly yellow needle-like crystals. freely soluble in ethanol, ether and hot water. brown in color with ferric chloride. |
|
Uses |
4-Hydroxycoumarin is a plant derived antioxidant, protecting against lipid peroxidation, as well as a potential inhibitor of HIV-1 Integrase. 4-Hydroxycoumarins are a very important class of biologically active drugs which are widely used as anticoagulants-Warfarin and Acenocoumarol. |
|
Definition |
ChEBI: 4-hydroxycoumarin is a hydroxycoumarin that is coumarin in which the hydrogen at position 4 is replaced by a hydroxy group. It is a conjugate acid of a 4-hydroxycoumarin(1-). |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 25, p. 677, 1960 DOI: 10.1021/jo01074a630 |
|
Flammability and Explosibility |
Notclassified |
|
Purification Methods |
Crystallise 4-hydroxycoumarin from water and dry it in a vacuum desiccator over pKEst Sicapent. [Beilstein 18/1 V 378.] |
InChI:InChI:1S/C9H6O3/c10-7-5-9(11)12-8-4-2-1-3-6(7)8/h1-5,10H
-
A series of novel coumarin-based hydroxa...
A series of novel hybrids has been synth...
A one pot three component, copper cataly...
-
An improved one-pot synthesis of 4-hydro...
A series of novel 4-(4-amino phenyl) mor...
Syntheses of 2,3-dimethyl-4H-furo[3,2-c]...
The synthesis of twenty-six 4-arylcoumar...
-
A number of compounds targeting differen...
Based on the microwave-assisted syntheti...
An efficient protocol for the synthesis ...
A series of novel 3-substituted-4-hydrox...
-
A series of substituted N-(2,3,4,6-tetra...
A convenient and simple method for the s...
A series of N2,N2′-bis[4-hydroxycoumarin...
A highly stereoselective one-pot procedu...
Selenium assisted carbonylation of alkyl...
4-Hydroxycoumarins (4-hydroxy-2-oxo-2H-1...
An efficient I2/TBHP-mediated process fo...
The synthesis and reactivity of a series...
Selenium-assisted carbonylation of o-hyd...
A series of sulfonamides containing coum...
In this paper, a series of novel 4-subst...
A series of conjugates of podophyllotoxi...
Irradiation of chromone-2-carboxylic aci...
A series of lanthanide complexes [Ln(dpx...
Cancer patients frequently suffer from c...
An efficient protocol for synthesizing f...
The present invention discloses a series...
An efficient and mild catalytic deallyla...
C6H4(CH2CH2C(O)O)

![4-hydroxy[1]benzopyran-2-one](/upload/2023/6/277c9d94-4963-4191-825f-ebcd8108aea3.png)
4-hydroxy[1]benzopyran-2-one

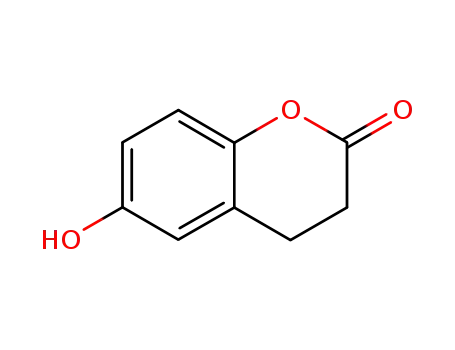
6-hydroxy-3,4-dihydrocoumarin

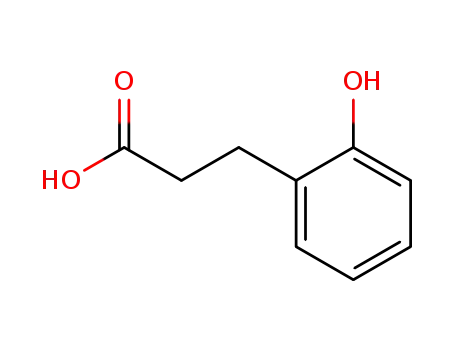
3-(2-hydroxyphenyl)propionic acid

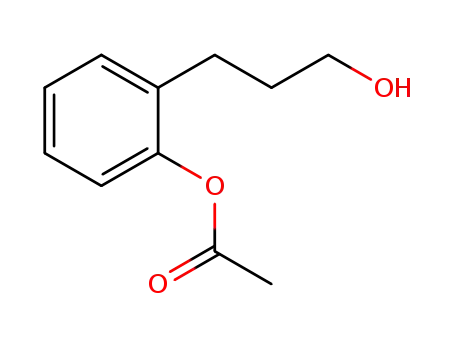
2-(3-hydroxypropyl)phenyl acetate

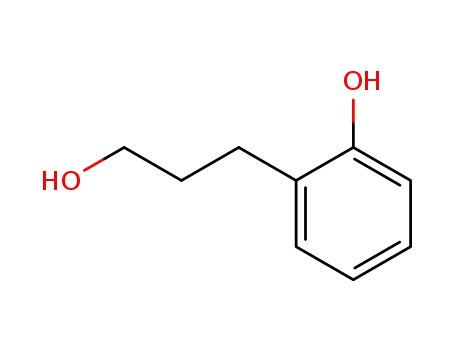
2-(3-hydroxypropyl)phenol

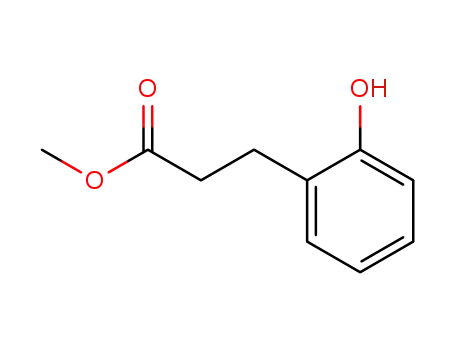
methyl 3-(2-hydroxyphenyl)propionate
| Conditions | Yield |
|---|---|
|
With
sodium nitrate; dipotassium hydrogenphosphate; magnesium sulfate heptahydrate; potassium chloride; iron(II) sulfate; Sucrose;
In
water; dimethyl sulfoxide;
at 28 ℃;
for 96h;
pH=5;
Reagent/catalyst;
Time;
Kinetics;
Microbiological reaction;
|
15 %Chromat. 8.4 %Chromat. 38 %Chromat. 4.6 %Chromat. 24 %Chromat. 10 %Chromat. |
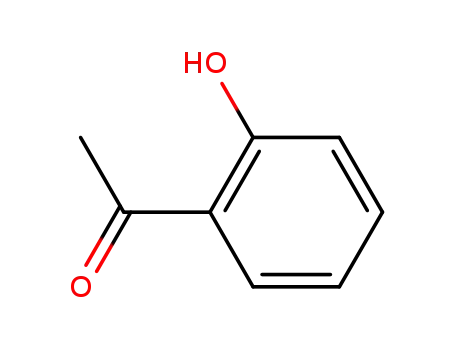
o-hydroxyacetophenone

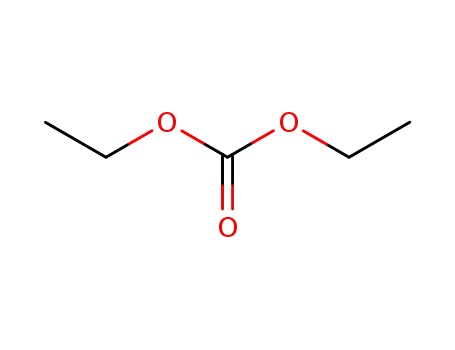
Diethyl carbonate

![4-hydroxy[1]benzopyran-2-one](/upload/2023/6/277c9d94-4963-4191-825f-ebcd8108aea3.png)
4-hydroxy[1]benzopyran-2-one
| Conditions | Yield |
|---|---|
|
With
sodium hydride;
In
toluene;
at 0 ℃;
for 4.5h;
Reflux;
|
95% |
|
With
sodium hydride;
In
toluene;
at 100 ℃;
for 3h;
|
85% |
|
o-hydroxyacetophenone;
With
sodium hydride;
In
toluene; mineral oil;
Diethyl carbonate;
In
toluene; mineral oil;
Reflux;
With
hydrogenchloride;
In
water;
|
85% |
|
o-hydroxyacetophenone;
With
sodium hydride;
In
toluene;
at 0 ℃;
for 0.333333h;
Inert atmosphere;
Diethyl carbonate;
at 110 ℃;
for 4h;
Inert atmosphere;
|
81% |
|
o-hydroxyacetophenone;
With
sodium hydride;
In
toluene; mineral oil;
at 20 ℃;
for 0.5h;
Inert atmosphere;
Diethyl carbonate;
In
toluene; mineral oil;
for 3h;
Reflux;
|
68% |
|
o-hydroxyacetophenone;
With
sodium hydride;
In
toluene;
at 20 ℃;
for 0.5h;
Cooling with ice;
Diethyl carbonate;
In
toluene;
for 4h;
Reflux;
|
65% |
|
With
sodium hydroxide;
In
benzene;
|
50% |
|
With
sodium;
for 1.16667h;
Heating;
Inert atmosphere;
|
45% |
|
With
sodium hydride;
|
|
|
With
sodium hydride;
In
toluene;
at 110 ℃;
|
|
|
With
sodium hydride;
In
toluene;
at 105 ℃;
for 3h;
Inert atmosphere;
|
|
|
With
sodium hydride;
In
toluene; mineral oil;
at 0 - 80 ℃;
for 2h;
|
|
|
With
sodium hydride;
In
mineral oil;
at 0 ℃;
for 5h;
Reflux;
|
|
|
o-hydroxyacetophenone;
With
sodium hydride;
In
toluene;
for 0.25h;
Inert atmosphere;
Cooling with ice;
Diethyl carbonate;
In
toluene;
at 20 ℃;
for 5.5h;
Inert atmosphere;
Reflux;
|
|
|
With
sodium hydride;
at 100 ℃;
for 3h;
|
|
|
o-hydroxyacetophenone;
With
sodium hydride;
In
toluene; mineral oil;
for 0.166667h;
Inert atmosphere;
Diethyl carbonate;
In
toluene; mineral oil;
for 3.5h;
Reflux;
|
|
|
With
sodium hydride;
In
toluene;
|
|
|
With
sodium hydride;
|
|
|
With
sodium hydride;
at 0 - 200 ℃;
for 2.5h;
|
|
|
With
sodium hydride;
In
toluene;
Inert atmosphere;
Reflux;
|
|
|
o-hydroxyacetophenone;
With
sodium hydride;
In
toluene;
at 0 ℃;
for 0.25h;
Inert atmosphere;
Diethyl carbonate;
In
toluene;
Inert atmosphere;
|
|
|
With
sodium hydride;
In
toluene;
at 100 ℃;
for 3h;
|
|
|
o-hydroxyacetophenone;
With
sodium hydride;
In
toluene;
at 0 ℃;
for 0.25h;
Diethyl carbonate;
In
toluene;
at 0 - 120 ℃;
|
|
|
With
sodium;
Yield given. Multistep reaction;
1.) reflux, 4 h;
|
|
|
With
sodium hydride;
at 0 ℃;
Cooling with ice;
|
|
|
With
sodium hydride;
In
toluene;
at 0 - 100 ℃;
Inert atmosphere;
|
|
|
With
sodium hydride;
In
toluene;
|
|
|
With
sodium methylate;
In
diethyl ether;
for 5h;
Heating;
|
|
|
With
sodium hydride;
at 0 - 110 ℃;
for 4h;
|
|
|
With
sodium hydride;
In
toluene;
at 0 - 120 ℃;
for 12h;
|
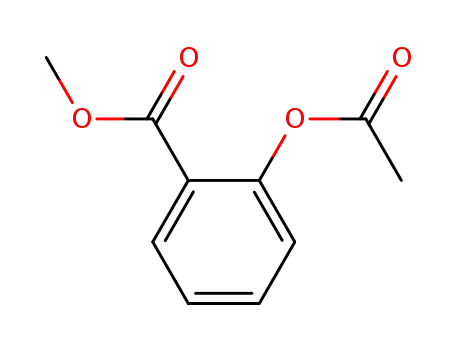
methyl acetylsalicylate
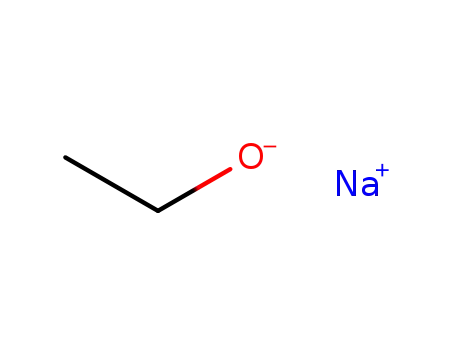
sodium ethanolate

o-hydroxyacetophenone

Diethyl carbonate
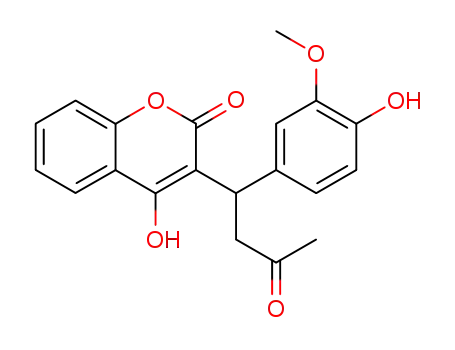
4-hydroxy-3-[1-(4-hydroxy-3-methoxy-phenyl)-3-oxo-butyl]-coumarin
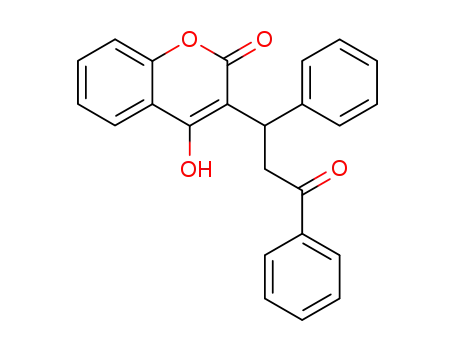
4-hydroxy-3-(3-oxo-1,3-diphenylpropyl)-2H-chromen-2-one
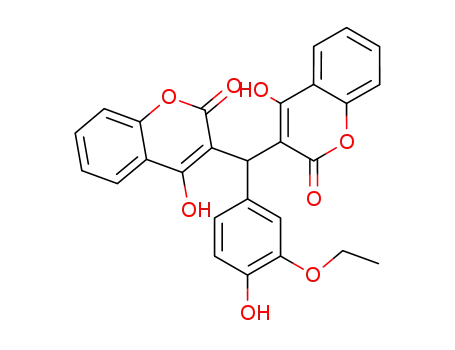
(3-ethoxy-4-hydroxy-phenyl)-bis-(4-hydroxy-2-oxo-2H-chromen-3-yl)-methane
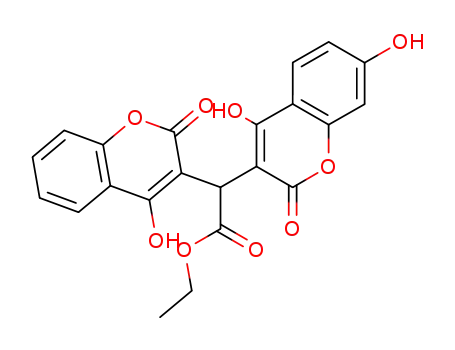
(4,7-dihydroxy-2-oxo-2H-chromen-3-yl)-(4-hydroxy-2-oxo-2H-chromen-3-yl)-acetic acid ethyl ester
CAS:138071-82-6
CAS:57280-22-5
CAS:92-66-0
CAS:445-27-2