- Language:English
- English
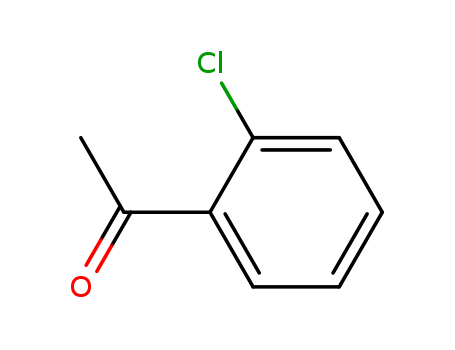

CasNo: 2142-68-9
Molecular Formula: C8H7ClO
Appearance: clear light yellow to amber liquid
|
Chemical Properties |
clear light yellow to amber liquid |
|
Uses |
2''-Chloroacetophenone (cas# 2142-68-9) is a compound useful in organic synthesis. |
|
General Description |
2′-Chloroacetophenone undergoes stereoselective reduction to (R)-2′-chloro-1-phenyl-ethanol by Saccharomyces cerevisiae B5. It is commonly used as lacrimator. |
-
A selective oxidation of secondary alcoh...
RuO2/V2O5 nanocatalyst has been prepared...
Ruthenium(II) complexes, [RuCl(L)(CO)(PP...
An efficient room temperature catalytic ...
A novel deep eutectic solvent supported ...
-
A practical and general iron-catalyzed d...
A highly ordered organic-inorganic hybri...
Ano-quinone methide (o-QM) featuring an ...
Four ruthenium(II) complexes 1—4 [RN=CH-...
C14H28O7*C8H7N2O(1+)*BF4(1-)

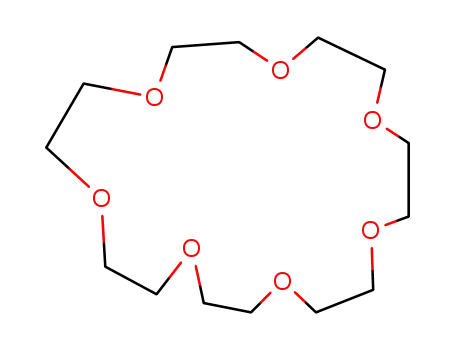
1,4,7,10,13,16,10-heptaoxacyclohenicosane

1-(2-chlorophenyl)ethanone
| Conditions | Yield |
|---|---|
|
In
1,2-dichloro-ethane;
at 20 ℃;
Rate constant;
Kinetics;
Thermodynamic data;
var. crown ethers, ΔH(excit.), ΔS(excit.);
|
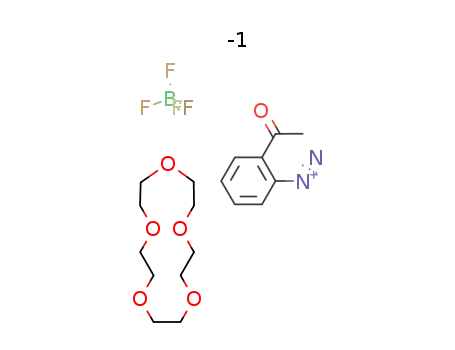
o-acetylbenzenediazonium tetrafluoroborate

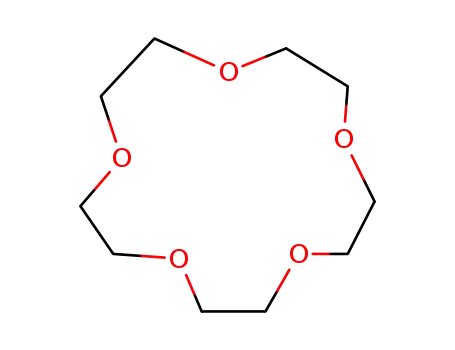
15-crown-5


1-(2-chlorophenyl)ethanone
| Conditions | Yield |
|---|---|
|
In
1,2-dichloro-ethane;
at 20 ℃;
Rate constant;
Kinetics;
Thermodynamic data;
var. crown ethers, ΔH(excit.), ΔS(excit.);
|
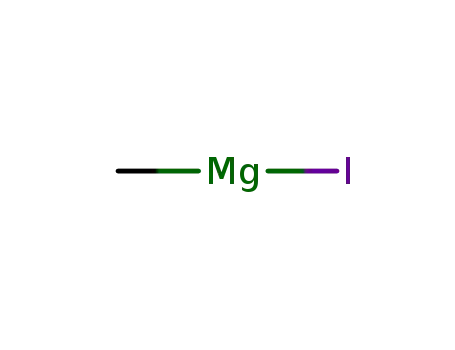
methyl magnesium iodide
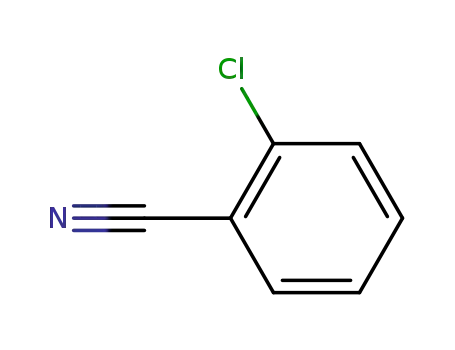
2-Chlorobenzonitrile
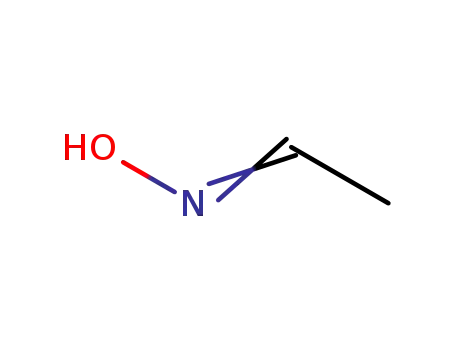
Acetaldehyde oxime
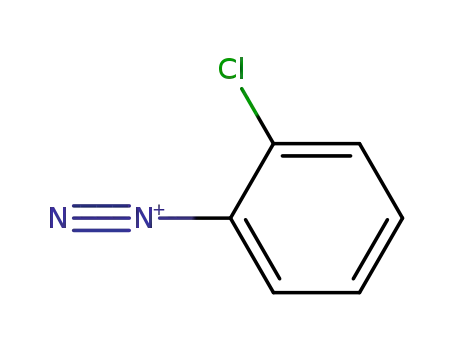
2-chlorobenzenediazonium
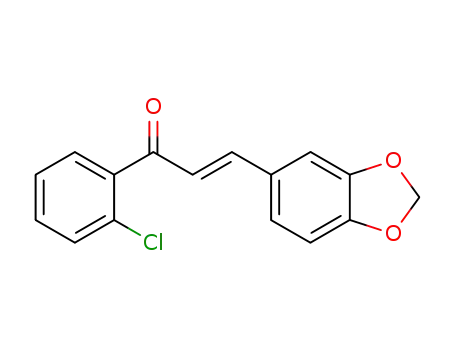
(E)-1-(2-chlorophenyl)-3-(3,4-methylenedioxy-phenyl)prop-2-en-1-one
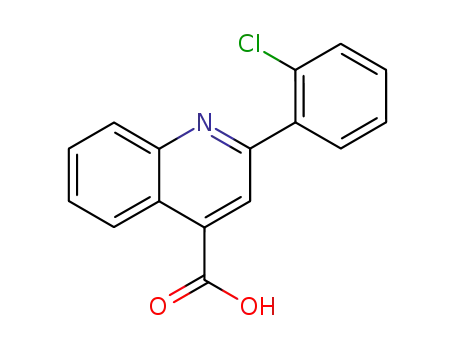
2-(2-chlorophenyl)-quinoline-4-carboxylic acid
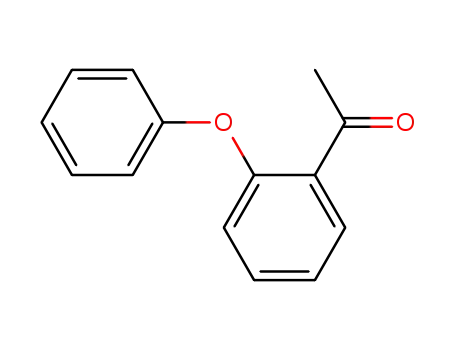
1-(2-phenoxyphenyl)ethan-1-one
1-(2-chlorophenyl)ethanone oxime
CAS:38083-17-9
CAS:770-12-7
CAS:445-27-2
CAS:577-16-2