- Language:English
- English
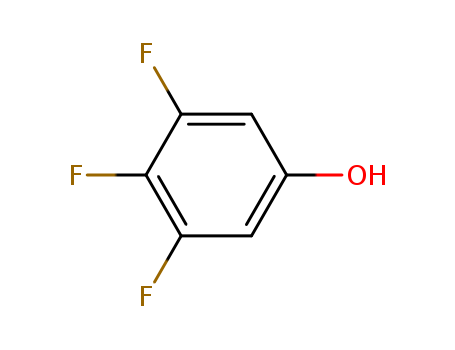

CasNo: 99627-05-1
Molecular Formula: C6H3F3O
Appearance: white to light yellow crystal powder
|
Chemical Properties |
white to light yellow crystal powder |
|
Uses |
3,4,5-Trifluorophenol may be used in the synthesis of 3,4,5-trifluorophenoxymethyl-substituted polystyrene. |
|
General Description |
3,4,5-Trifluorophenol is a halo-substituted phenol. It participates in the synthesis of difluorooxymethylene-bridged liquid crystals. |
|
Flammability and Explosibility |
Notclassified |
InChI:InChI=1/C6H3F3O/c7-4-1-3(10)2-5(8)6(4)9/h1-2,10H
The invention discloses a synthesis tech...
The invention discloses a synthesis meth...
A novel and efficient strategy for the i...
The invention provides a preparation met...
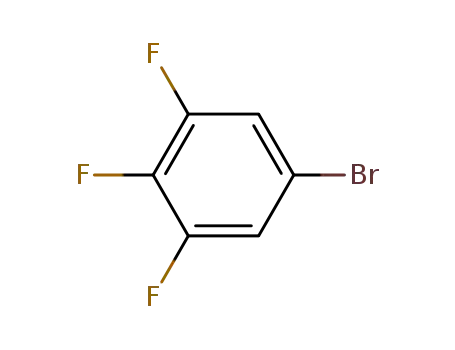
3,4,5-trifluoro-1-bromobenzene

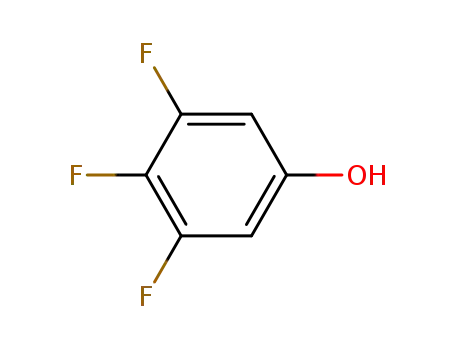
3,4,5-trifluorophenol
| Conditions | Yield |
|---|---|
|
With
sulfolane; potassium hydroxide;
In
water;
at 150 ℃;
for 10h;
under 7500.75 Torr;
Inert atmosphere;
Autoclave;
|
81.3% |
|
Multi-step reaction with 2 steps
1: 1,10-Phenanthroline; copper(I) bromide; potassium carbonate / 5 h / 80 °C / Inert atmosphere
2: sulfuric acid / water / 2 h / 90 °C
With
1,10-Phenanthroline; sulfuric acid; potassium carbonate; copper(I) bromide;
In
water;
|
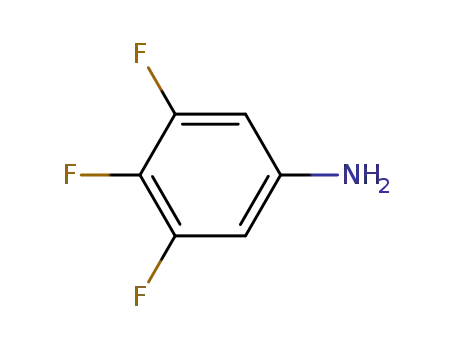
3,4,5-trifluoro aniline


3,4,5-trifluorophenol
| Conditions | Yield |
|---|---|
|
3,4,5-trifluoro aniline;
With
nitrosylsulfuric acid; sulfuric acid;
In
water;
at -5 - 70 ℃;
for 1.5h;
Autoclave;
With
sulfuric acid; copper(II) sulfate;
In
water;
Solvent;
Reagent/catalyst;
Reflux;
|
90.2% |
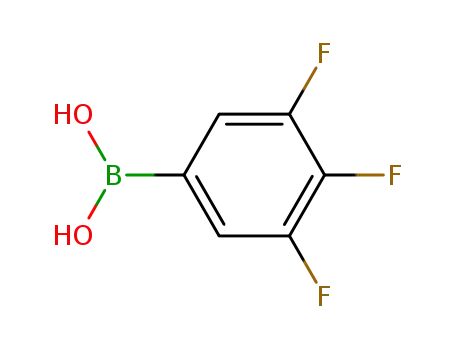
3,4,5-trifluorophenylboronic acid
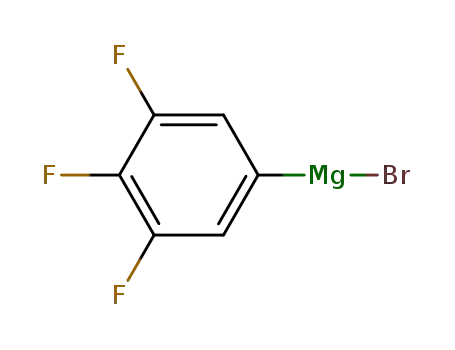
3,4,5-trifluorophenylmagnesium bromide
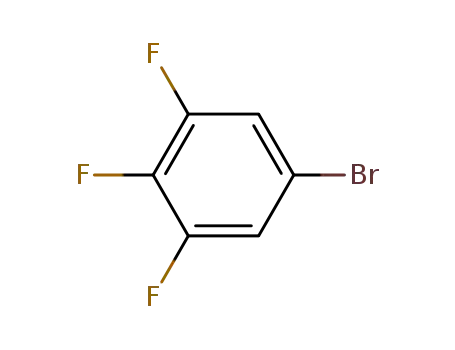
3,4,5-trifluoro-1-bromobenzene

3,4,5-trifluoro aniline
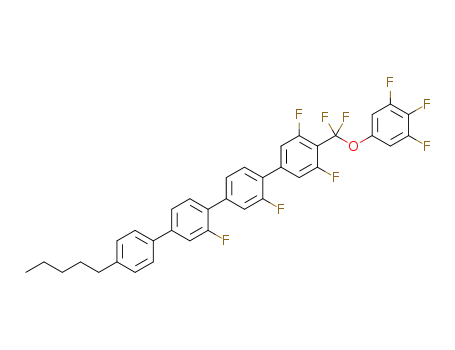
4-[difluoro(3,4,5-trifluorophenoxy)methyl]-4'''-n-pentyl-2',2'',3,5-tetrafluoro-1,1',4',1'',4'',1'''-quaterphenyl
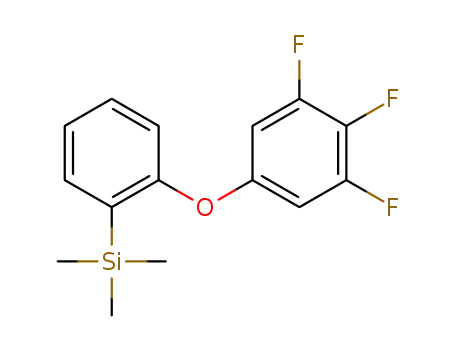
trimethyl(2-(3,4,5-trifluorophenoxy)phenyl)silane
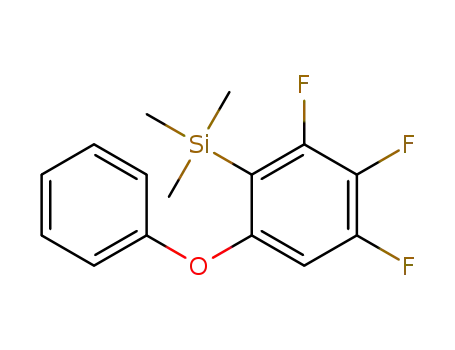
trimethyl(2,3,4-trifluoro-6-phenoxyphenyl)silane
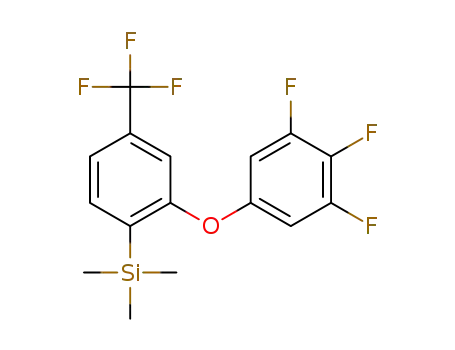
4-trifluoromethyl-2-((3,4,5-trifluorophenoxy)phenyl)trimethylsilane
CAS:863329-66-2
CAS:24424-99-5
CAS:946511-97-3