- Language:English
- English
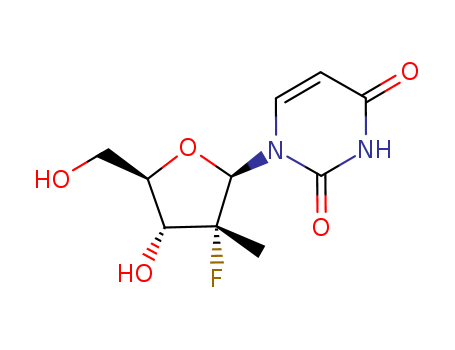

CasNo: 863329-66-2
Molecular Formula: C10H13FN2O5
Appearance: Off-white solid
|
Description |
(2'R)-2'-Deoxy-2'-fluoro-2'-methyluridine / PSI-6206 is the deaminated metabolite of β-D-2'-Deoxy-2'-fluoro-2'-C-methylcytidine, an effective inhibitor of hepatitis C virus (HCV) RNA polymerase. |
|
Uses |
2'-deoxy-2'-fluoro-2'-C-methyluridine is the deaminated metabolite of β-D-2''-Deoxy-2''-fluoro-2''-C-methylcytidine, an effective inhibitor of hepatitis C virus (HCV) replication in vitro. |
|
Biological Activity |
In contrast, the average concentration of 2'-deoxy-2'-fluoro-2'-C-methyluridine in CSF samples after i.v. administration was 1.5 ± 0.8 μM. However, the concentration of 2'-deoxy-2'-fluoro-2'-C-methyluridine in CSF samples was below the limit of detection (≤0.38 μM) following oral administration. |
Isomeric SMILES: C[C@]1([C@@H]([C@H](O[C@H]1N2C=CC(=O)NC2=O)CO)O)F
InChIKey: ARKKGZQTGXJVKW-VPCXQMTMSA-N
InChI: InChI=1S/C10H13FN2O5/c1-10(11)7(16)5(4-14)18-8(10)13-3-2-6(15)12-9(13)17/h2-3,5,7-8,14,16H,4H2,1H3,(H,12,15,17)/t5-,7-,8-,10-/m1/s1
The total bioavailability, taking into account the parent drug and its deaminated metabolite 2′-deoxy-2′-fluoro-2′-C-methyluridine (PSI-6206),
The invention discloses a preparation me...
The invention belongs to the technical f...
As part of our SAR study of the 2 ′-deoxy-2 ′-fluoro-2 ′- C-methyl class of nucleosides, we prepared the cyclopentyl carbocyclic uridine analog 11 and its phosphoramidate prodrug 15. Both 11 and 15 were shown not to inhibit HCV replication.
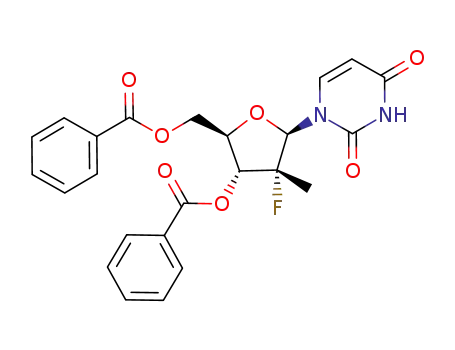
(2‘R)-2‘-deoxy-2’-fluoro-2-methyluridine-3’,5’-dibenzoate

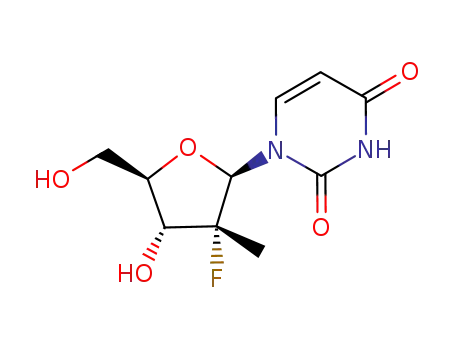
2'-deoxy-2'-fluoro-2'-methyluridine
| Conditions | Yield |
|---|---|
|
With ammonia; In methanol; at 20 ℃;
|
100% |
|
With ammonia; In methanol; at 20 ℃; for 17h;
|
93% |
|
With methanol; triethylamine; for 53h; Reflux;
|
93.8% |
|
(2‘R)-2‘-deoxy-2’-fluoro-2-methyluridine-3’,5’-dibenzoate; With N-butylamine; at 45 - 50 ℃;
With methanol; In tert-butyl methyl ether; at 60 - 70 ℃;
|
90.7% |
|
With sodium methylate; In methanol; Reagent/catalyst; Reflux;
|
82.6% |
|
With ammonia; In methanol; at 0 - 15 ℃; for 27h;
|
78% |
|
With ammonia; In methanol; at 0 - 15 ℃; for 27h;
|
78% |
|
With ammonia; In methanol; at 0 - 15 ℃; for 27h;
|
78% |
|
With methanol; ammonia; at 20 ℃; for 30h;
|
62% |
|
With ammonia; In methanol; at 0 - 20 ℃; for 18.5h;
|
60% |
|
With methanol; ammonia; at 0 - 20 ℃;
|
60% |
|
(2‘R)-2‘-deoxy-2’-fluoro-2-methyluridine-3’,5’-dibenzoate; With sodium methylate; In methanol; at -10 - 20 ℃; for 2h; Inert atmosphere; Large scale;
With sulfuric acid; In methanol; at -10 ℃; Large scale;
|
59.15% |
|
(2‘R)-2‘-deoxy-2’-fluoro-2-methyluridine-3’,5’-dibenzoate; With sodium methylate; In methanol; at -10 - 20 ℃; for 2h; Inert atmosphere; Large scale;
With sulfuric acid; In methanol; at -10 ℃; Large scale;
|
59.15% |
|
With sodium methylate; In methanol; Temperature; Reflux;
|
|
|
With methanol; ammonia; at 0 - 15 ℃; for 27h;
|
35 g |
|
With monomethanolamine; at 25 - 30 ℃; for 24h;
|
3 g |
|
With sodium methylate; In methanol; Reflux;
|
|
|
With ammonia; at 0 - 15 ℃; for 20h;
|
55 g |
|
With ammonia; In methanol;
|
(2'R)-2'-deoxy-2'-fluoro-2'-methyluridine-3',5'-dibenzoate


2'-deoxy-2'-fluoro-2'-methyluridine
| Conditions | Yield |
|---|---|
|
With ammonia; In methanol; at 0 - 15 ℃; for 27h;
|
94% |
|
With methanol; triethylamine; for 53h; Reflux;
|
92.6% |

(2‘R)-2‘-deoxy-2’-fluoro-2-methyluridine-3’,5’-dibenzoate
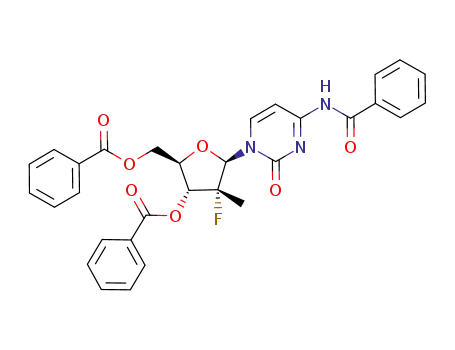
(2R,3R,4R,5R)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4-fluoro-4-methyltetrahydrofuran-3-yl benzoate
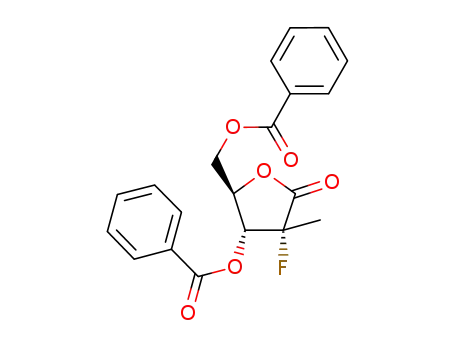
((2R,3R,4R)-3-benzoyloxy-4-fluoro-4-methyl-5-oxo-tetrahydrofuran-2-yl)methyl benzoate
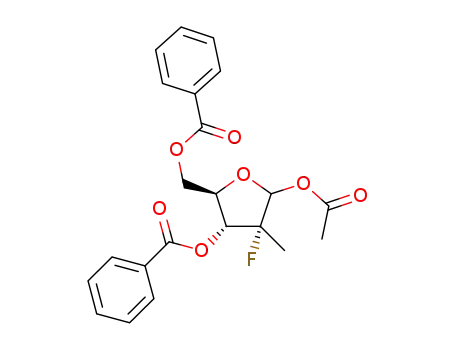
(2R,3R,4R,5R)-5-acetoxy-2-((benzoyloxy)methyl)-4-fluoro-4-methyl-tetrahydrofuran-3-yl benzoate
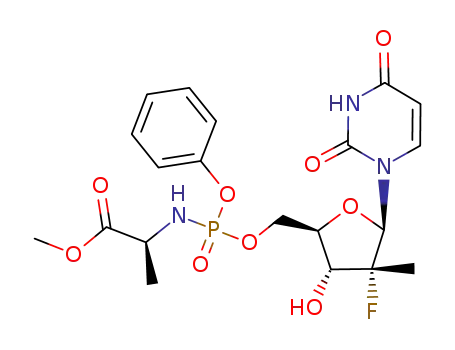
(S)-2-{[(2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-ylmethoxy](phenoxy)phosphorylamino}propionic acid methyl ester
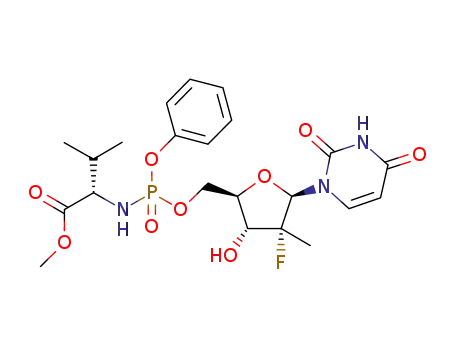
(S)-2-{[(2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-ylmethoxy](phenoxy)phosphorylamino}-3-methylbutyric acid methyl ester
(S)-2-{(4-bromophenoxy)-[(2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-ylmethoxy]phosphorylamino}-3-methylbutyric acid methyl ester
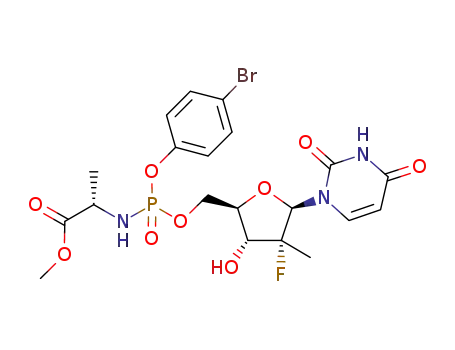
(S)-2-{(4-bromophenoxy)[(2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-ylmethoxy]phosphorylamino}propionic acid methyl ester
CAS:143062-84-4
CAS:770-12-7