- Language:English
- English
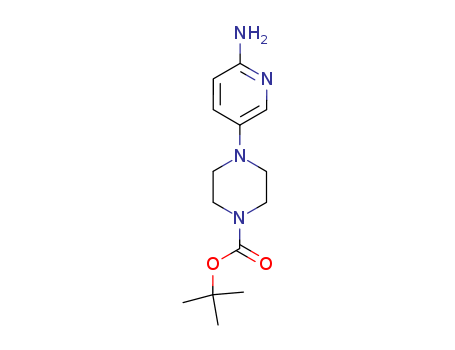

CasNo: 571188-59-5
Molecular Formula: C14H22N4O2
|
Physical Form |
Solid |
|
Uses |
4-(6-Amino-3-pyridyl)-1-Boc-piperazine is used as an organic chemical synthesis intermediate. |
InChI:InChI=1/C14H22N4O2/c1-14(2,3)20-13(19)18-8-6-17(7-9-18)11-4-5-12(15)16-10-11/h4-5,10H,6-9H2,1-3H3,(H2,15,16)
A copper-catalyzed 1,2-diol amination at...
A series of novel LEE011 derivatives con...
Multiple myeloma (MM) ranks second in ma...
The method adopts 4 - bromo 6 - nitropyr...
A novel class of heteroaryl compounds fo...
The present invention discloses a compou...
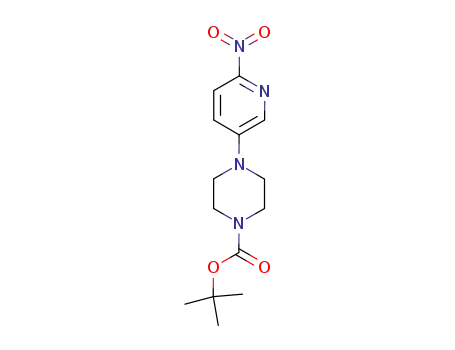
4-(6-nitropyridin-3-yl)-piperazine-1-carboxylic acid tert-butyl ester

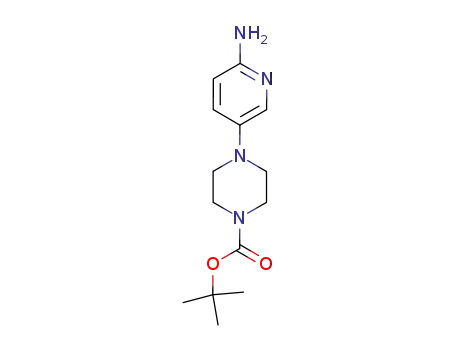
tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate
| Conditions | Yield |
|---|---|
|
With
hydrogen;
palladium on activated charcoal;
In
ethanol; water;
for 2h;
|
100% |
|
With
palladium on activated charcoal; hydrogen;
In
ethanol;
for 3h;
|
100% |
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
at 25 ℃;
for 4h;
under 2250.23 Torr;
Autoclave;
|
99.1% |
|
With
hydrogen;
palladium 10% on activated carbon;
In
ethanol;
at 20 ℃;
for 16h;
Product distribution / selectivity;
Inert atmosphere;
|
97% |
|
With
palladium 10% on activated carbon; hydrogen;
In
ethanol;
at 20 ℃;
for 16h;
|
97% |
|
With
palladium 10% on activated carbon; hydrogen;
In
ethanol;
at 20 ℃;
for 16h;
|
97% |
|
With
palladium 10% on activated carbon; hydrogen;
In
ethanol;
at 10 ℃;
for 16h;
Inert atmosphere;
|
97% |
|
4-(6-nitropyridin-3-yl)-piperazine-1-carboxylic acid tert-butyl ester;
With
iron(III) chloride hexahydrate;
In
ethanol;
at 80 ℃;
for 0.5h;
With
hydrazine hydrate;
In
ethanol;
at 80 ℃;
for 14h;
Concentration;
|
97.4% |
|
With
5%-palladium/activated carbon; hydrogen;
In
ethyl acetate;
at 42 - 47 ℃;
under 2585.81 Torr;
Inert atmosphere;
|
96% |
|
With
methanol; sodium sulfide; ammonium chloride;
In
water;
at 70 - 80 ℃;
for 2h;
Reagent/catalyst;
|
96.2% |
|
With
platinum on activated charcoal; hydrogen; sodium acetate;
In
methanol;
for 3h;
Autoclave;
Industrial scale;
|
96.8% |
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
at 20 ℃;
|
95% |
|
With
palladium on activated charcoal; hydrogen;
In
methanol; ethanol;
at 20 ℃;
for 2h;
|
95% |
|
With
5% Pd/C; hydrogen;
In
methanol;
at 50 ℃;
for 18h;
under 2585.81 Torr;
|
95.46% |
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
at 20 ℃;
for 12h;
under 2250.23 Torr;
|
95% |
|
With
5% Pd/C; hydrogen;
In
methanol;
at 50 ℃;
for 18h;
under 2585.81 Torr;
|
95.46% |
|
With
hydrogen;
palladium 10% on activated carbon;
In
ethanol; ethyl acetate;
under 760.051 Torr;
|
94% |
|
With
hydrogen;
palladium 10% on activated carbon;
In
ethanol; ethyl acetate;
for 24h;
|
93% |
|
With
hydrogen;
palladium 10% on activated carbon;
In
methanol; water;
at 19 - 54 ℃;
for 0.25h;
under 1551.49 - 2327.23 Torr;
Inert atmosphere;
|
93.4% |
|
With
palladium 10% on activated carbon; hydrogen;
In
ethanol; ethyl acetate;
at 20 ℃;
for 12h;
|
93% |
|
With
palladium on activated charcoal; hydrogen;
In
methanol;
at 20 ℃;
|
93.1% |
|
With
palladium 10% on activated carbon; hydrogen;
In
ethanol; ethyl acetate;
at 20 ℃;
|
92% |
|
With
palladium 10% on activated carbon; hydrogen;
In
ethanol;
at 100 ℃;
for 0.00833333h;
under 11251.1 Torr;
Concentration;
Temperature;
Solvent;
Pressure;
|
92.03% |
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
at 20 ℃;
|
92% |
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
at 20 ℃;
for 4h;
|
90.88% |
|
With
acetic acid; zinc;
In
water;
at 10 - 20 ℃;
for 2h;
|
89% |
|
With
palladium 10% on activated carbon;
In
methanol;
at 20 ℃;
for 10h;
|
89% |
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
|
88% |
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
|
88% |
|
With
10% Pd/C; hydrogen;
In
methanol;
at 20 ℃;
|
85% |
|
With
iron; ammonium chloride;
In
ethanol;
at 70 ℃;
for 6h;
|
85% |
|
With
ethanol; iron; ammonium chloride;
at 70 ℃;
for 6h;
|
85% |
|
With
iron; ammonium chloride;
In
ethanol;
at 70 ℃;
Inert atmosphere;
|
85% |
|
With
iron; ammonium chloride;
In
ethanol;
at 70 ℃;
for 6h;
|
85% |
|
With
ammonium chloride;
In
ethanol;
at 70 ℃;
for 6h;
|
85% |
|
With
iron; ammonium chloride;
In
ethanol;
at 70 ℃;
for 6h;
|
85% |
|
With
hydrogen;
palladium(II) hydroxide/carbon;
In
isopropyl alcohol;
|
84% |
|
With
hydrogen;
nickel;
In
methanol;
for 5h;
under 2585.74 Torr;
|
83% |
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
at 20 ℃;
|
83% |
|
With
hydrogen;
In
methanol; water;
for 5h;
under 2585.81 Torr;
|
83% |
|
With
hydrogen;
In
methanol; water;
under 2585.81 Torr;
|
83% |
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
at 20 ℃;
for 24h;
under 760.051 Torr;
|
82.5% |
|
With
palladium 10% on activated carbon; hydrogen;
In
ethanol; ethyl acetate;
at 20 ℃;
for 5h;
under 750.075 Torr;
|
80% |
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
for 3h;
|
55.39% |
|
With
cyclohexane;
palladium 10% on activated carbon;
In
ethanol;
at 85 ℃;
for 60h;
|
|
|
With
hydrogen;
palladium 10% on activated carbon;
In
ethanol; ethyl acetate;
at 20 ℃;
|
|
|
With
hydrogen;
palladium on carbon;
In
methanol;
for 4h;
|
|
|
With
10% palladium on activated carbon; hydrogen;
In
methanol;
|
|
|
With
hydrogen;
Raney-Nickel;
In
methanol;
at 20 ℃;
for 4h;
|
|
|
With
palladium 10% on activated carbon; hydrogen;
In
ethanol;
for 2h;
|
|
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
for 3h;
Inert atmosphere;
|
|
|
With
ammonium chloride; zinc;
In
methanol;
at 20 ℃;
for 1h;
|
26 g |
|
With
hydrogen;
In
methanol;
at 40 ℃;
Temperature;
|
|
|
With
hydrogen;
In
methanol;
at 20 ℃;
for 4h;
Reagent/catalyst;
|
7.68 g |
|
With
palladium 10% on activated carbon; hydrogen;
In
ethanol; water;
at 20 ℃;
for 16h;
|
|
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
at 20 ℃;
|
|
|
With
palladium on activated charcoal; hydrogen; acetic acid;
In
methanol;
at 25 ℃;
for 4h;
|
|
|
With
palladium on activated charcoal; hydrogen;
In
methanol; ethyl acetate;
at 20 ℃;
for 2h;
|
792 mg |
|
With
palladium on activated charcoal; hydrogen;
In
ethanol;
at 25 ℃;
for 3h;
|
|
|
With
hydrazine hydrate; FeO(OH)/C;
In
ethanol;
Reflux;
|
|
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
at 20 ℃;
for 16h;
|
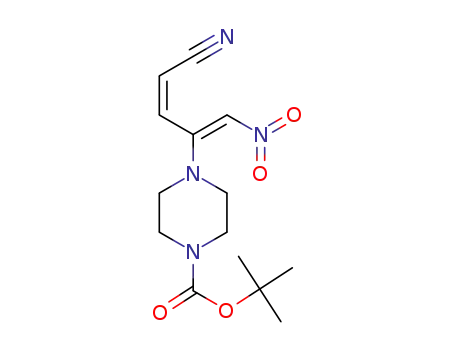
1-(5-nitro-2Z,4Z-pentadienenitrile-4-yl)-4-tert-butoxycarbonylpiperazine


tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate
| Conditions | Yield |
|---|---|
|
With
5%-palladium/activated carbon; hydrogen; ammonium chloride;
In
methanol;
at 40 - 45 ℃;
under 1500.15 - 2250.23 Torr;
Temperature;
Reagent/catalyst;
Pressure;
Solvent;
Autoclave;
|
95.3% |

4-(6-nitropyridin-3-yl)-piperazine-1-carboxylic acid tert-butyl ester
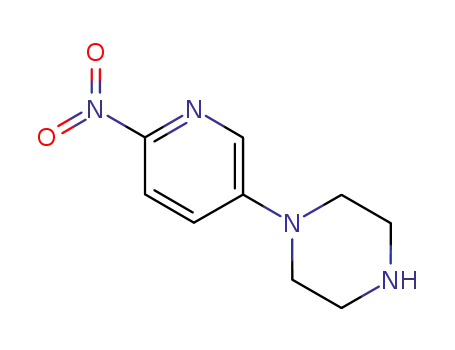
1-(6-nitropyridin-3-yl)piperazine
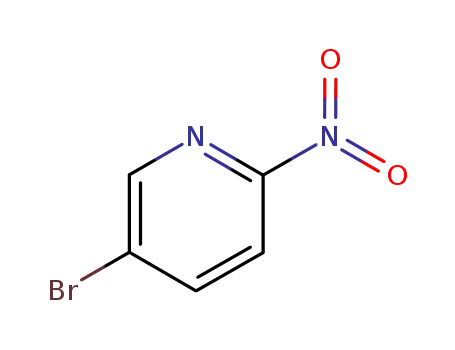
5-bromo2-nitropyridine
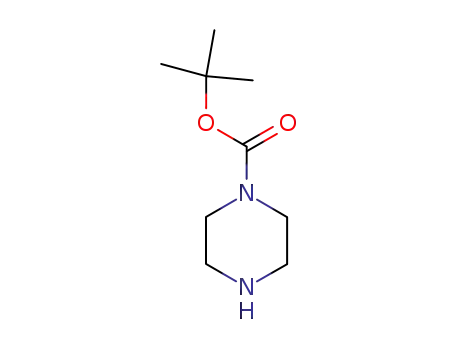
1-t-Butoxycarbonylpiperazine
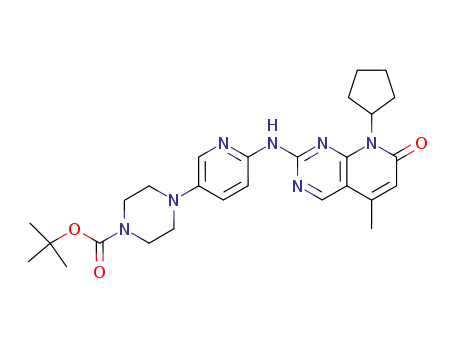
4-[6-(8-cyclopentyl-5-methyl-7-oxo-7,8-dihydropyrido[2,3-d]pyrimidin-2-ylamino)pyridin-3-yl]piperazine-1-carboxylic acid tert-butyl ester
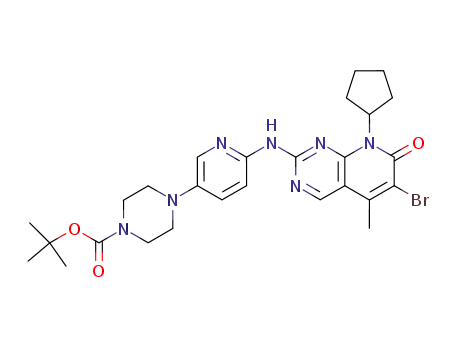
tert?butyl 4?(6?((6?bromo?8?cyclopentyl?5?methyl?7?oxo?7,8?dihydropyrido[2,3?d]pyrimidin?2?yl)amino)pyridin?3?yl)piperazine?1?carboxylate
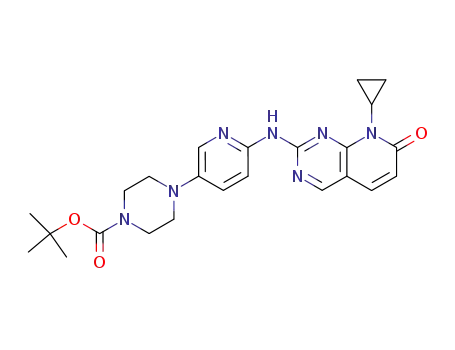
4-[6-(8-cyclopropyl-7-oxo-7,8-dihydropyrido[2,3-d]pyrimidin-2-ylamino)pyridin-3-yl]piperazine-1-carboxylic acid tert-butyl ester
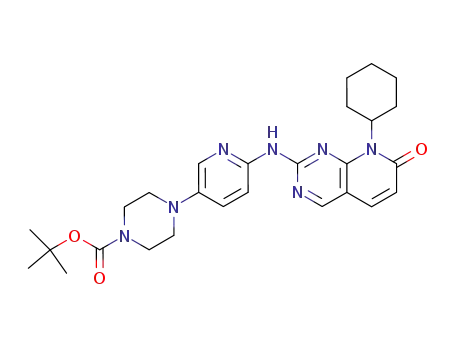
4-[6-(8-cyclohexyl-7-oxo-7,8-dihydropyrido[2,3-d]pyrimidin-2-ylamino)pyridin-3-yl]piperazine-1-carboxylic acid tert-butyl ester
CAS:138071-82-6
CAS:38083-17-9
CAS:946511-97-3
CAS:198904-85-7