- Language:English
- English
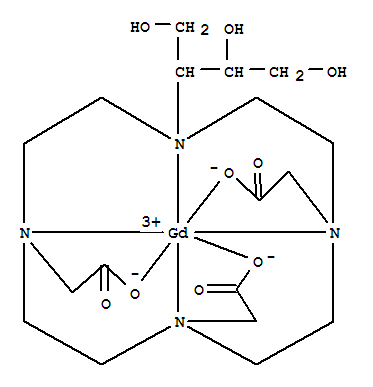

CasNo: 138071-82-6
Molecular Formula: C18H31 Gd N4 O9
|
Mechanism of action |
In the central nervous system, Gadobutrol works by highlighting any areas with disrupted blood brain barrier (BBB) and/or abnormal vascularity. In breast tissue, Gadobutrol exposes the presence and extent of malignant breast disease. |
|
Waste Disposal |
Expired or waste pharmaceuticals shall carefully take into consideration applicable DEA, EPA, and FDA regulations. It is not appropriate to dispose by flushing the pharmaceutical down the toilet or discarding to trash. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator. |
InChI:InChI=1/C18H34N4O9.Ga/c23-12-14(15(25)13-24)22-7-5-20(10-17(28)29)3-1-19(9-16(26)27)2-4-21(6-8-22)11-18(30)31;/h14-15,23-25H,1-13H2,(H,26,27)(H,28,29)(H,30,31);/q;+3/p-3/t14-,15-;/m1./s1
CAS:122795-43-1
CAS:3077-13-2
CAS:57280-22-5