- Language:English
- English
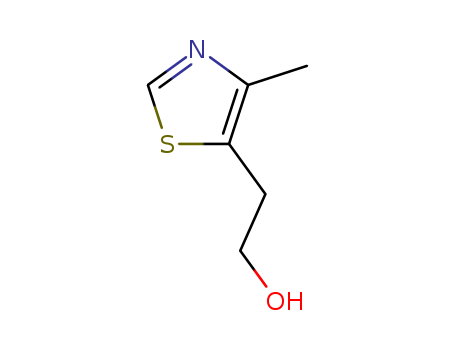

CasNo: 137-00-8
Molecular Formula: C6H9NOS
Appearance: Colorless to light yellow liquid
|
Content analysis |
It was determined by gas chromatography (GT-10-4) using a nonpolar column method. |
|
Toxicity |
GRAS (FEMA) |
|
Usage limit FEMA |
soft drinks, cold drinks, candy, baked goods, jelly, pudding, jelly sugar, seasonings, meat products, meat soup, milk and dairy products as well as cereal product: 55.0mg/kg. |
|
Application |
5-(2-hydroxyethyl)-4-methylthiazole is the precursor of the monomer MTZ. Used as temporarily allowable food spices; For nuts, dairy products, meat products, etc., as pharmaceutical intermediates; Used for the deployment of chocolate, milk and nuts |
|
Preparation |
It is obtained from the reduction of 4-methyl thiazole 5-ethyl acetate reduction with lithium aluminum hydride. It may be prepared by reduction of ethyl- 4-methylthiazole-5-acetate using LiAlH4; by condensation of thioformamide with bromoacetopropanol or with y,y-dichloro-y,ydiacetodipropyl ether. |
|
Occurrence |
Reported found in grilled and roasted beef, cognac, brandy, Finnish whiskey, cocoa, peanuts, beans and malt. |
|
Uses |
5-(2-Hydroxyethyl)-4-methylthiazole, a metabolic intermediate in the biosynthesis of thiamine, has been used in the synthesis of novel thiazolium halogenide ionic liquids. 5-(2-hydroxyethyl)-4-methylthiazole (HMT) is the analog compound of vitamin B1, and can be used as the counter-cation precursor. |
|
Definition |
ChEBI: A 1,3-thiazole that is thiazole substituted by a methyl group at position 4 and a 2-hydroxyethyl group at position 5. |
|
Taste threshold values |
Taste characteristics at 20 ppm: meaty, brothy, roasted, metallic and nutty. |
InChI:InChI=1/C6H9NOS/c1-5-6(2-3-8)9-4-7-5/h4,8H,2-3H2,1H3
Thiamine is extracted and then quantitatively split by sulfite treatment to give the ether-extractable 5-(2-hydroxyethyl)-4-methylthiazole. The thiazole was analyzed by GLC. Its purity and identity were confirmed by mass spectrometry and GLC retention time.
Thiaminase I catalyzes the decomposition...
The dark blue crystals were shown to have the composition Co-[5- (2-hydroxyethyl)-4-methylthiazole]2Br2 by microanal- ysis.
TBDMS (t-BuMe2Si, tert-butyldimethylsily...
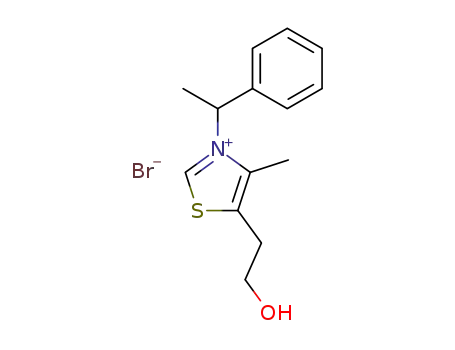
5-(2-hydroxy-ethyl)-4-methyl-3-(1-phenyl-ethyl)-thiazolium; bromide

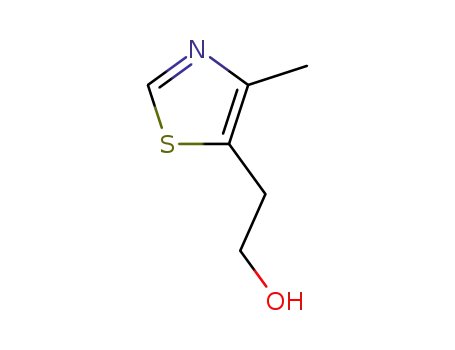
5-hydroxyethyl-4-methylthiazole

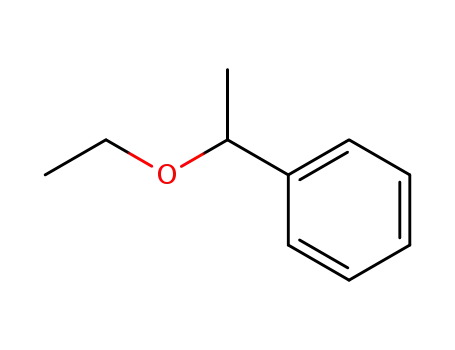
1-ethoxy-1-phenylethane
| Conditions | Yield |
|---|---|
|
|
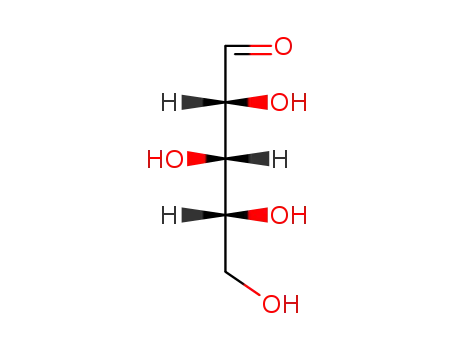
D-xylose

vitamin B1

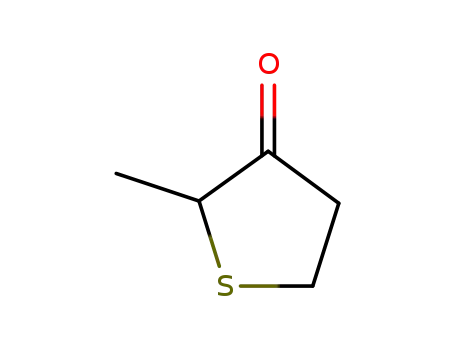
4,5-Dihydro-2-methylthiophen-3-(2H)-on


5-hydroxyethyl-4-methylthiazole

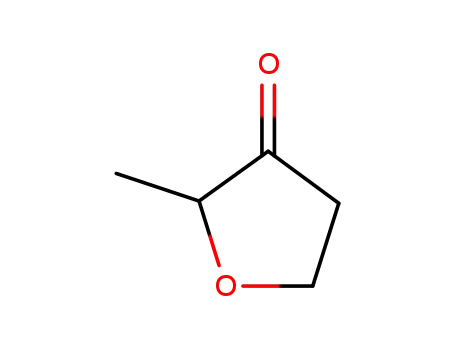
2-methyltetrahydrofuran-3-one

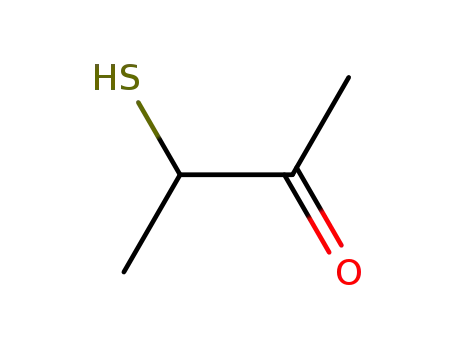
3-mercapto-2-butanone


3-mercaptopentan-2-one

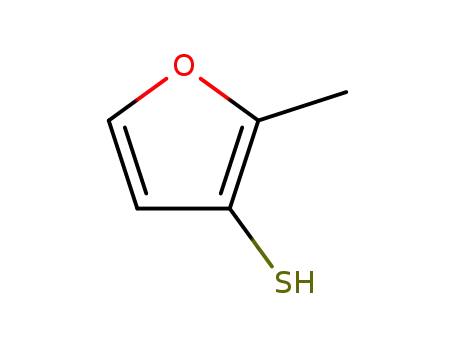
2-methylfuran-3-thiol
| Conditions | Yield |
|---|---|
|
With L-Cysteine; at 145 ℃; for 0.333333h; pH=7; aq. phosphate buffer;
|
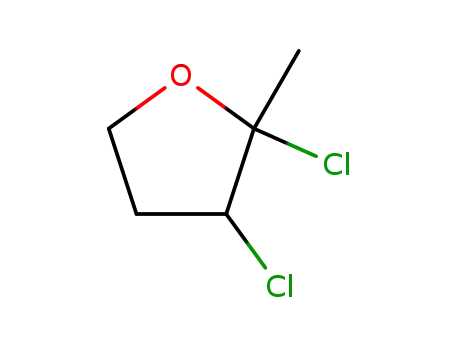
2,3-dichloro-2-methyl-tetrahydro-furan
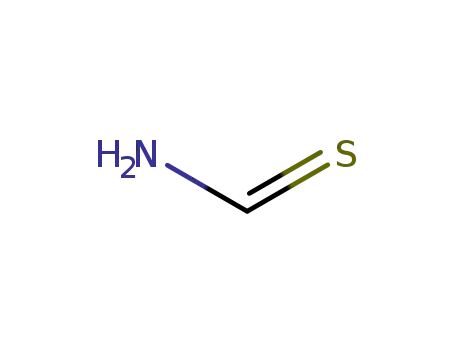
thiocarboxamide

2-ethoxy-3-chloro-2-methyl-tetrahydro-furan
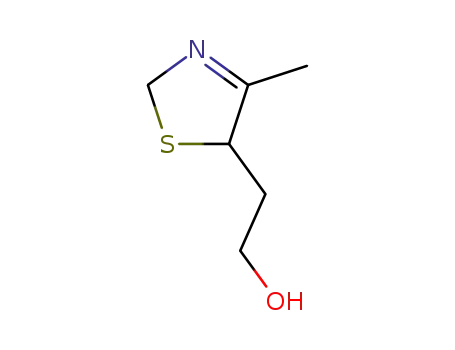
2-(4-methyl-2,5-dihydro-thiazol-5-yl)-ethanol
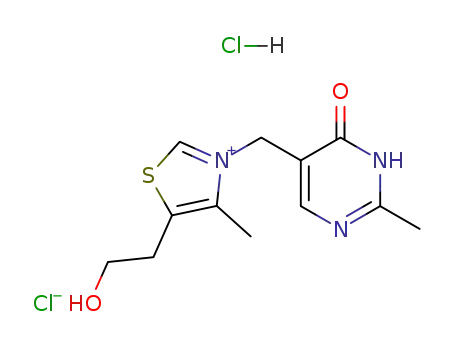
oxythiamine chloride hydrochloride
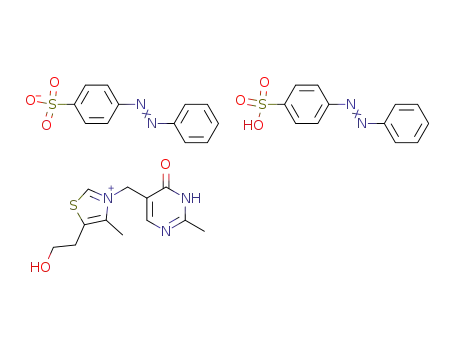
5-(2-hydroxy-ethyl)-4-methyl-3-(2-methyl-6-oxo-1,6-dihydro-pyrimidin-5-ylmethyl)-thiazolium; bis-(4-phenylazo-benzenesulfonate )
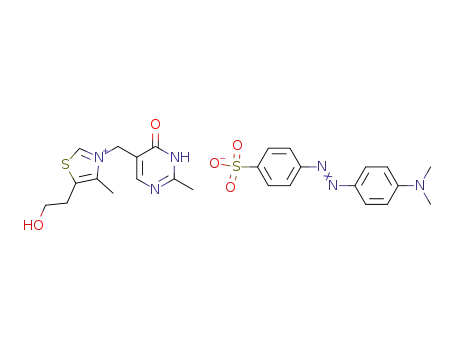
5-(2-hydroxy-ethyl)-4-methyl-3-(2-methyl-6-oxo-1,6-dihydro-pyrimidin-5-ylmethyl)-thiazolium; [4-(4-dimethylamino-phenylazo)-benzenesulfonate ]
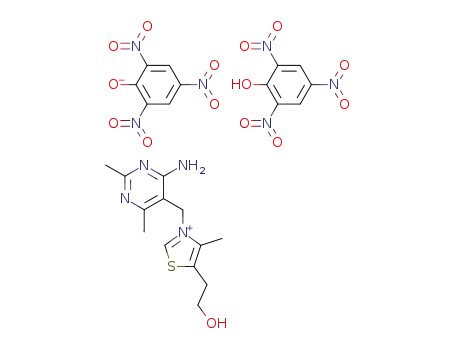
3-(4-amino-2,6-dimethyl-pyrimidin-5-ylmethyl)-5-(2-hydroxy-ethyl)-4-methyl-thiazolium; dipicrate
CAS:143062-84-4
CAS:71675-87-1
CAS:294-90-6
CAS:3959-07-7