- Language:English
- English
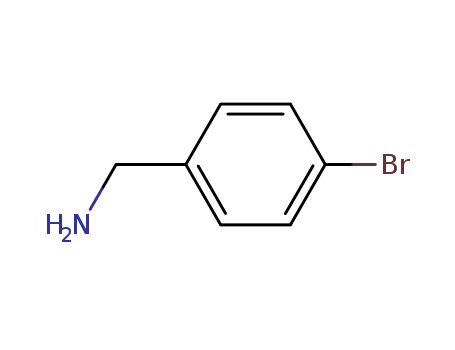

CasNo: 3959-07-7
Molecular Formula: C7H8 Br N
Appearance: Colorless to yellow clear liquid
|
Chemical Properties |
clear colorless to light yellow liquid |
|
Uses |
4-Bromobenzylamine (BrBeAI) may be used to synthesize 7-[(p-bromobenzyl)ureido]-7,8-dihydro-α-bisabolene.It may be used to synthesize the following 4-biphenylmethylamine derivatives:(4′-fluoro-4-biphenyl)methylamine(4′-methoxy-4-biphenyl)methylamine(2′-methoxy-4-biphenyl)methylamine(3′-cyano-4-biphenyl)methylamine. 4-Bromobenzylamine could increase the VB and CB of a perovskite, leading to a comprehensive increase of Voc, Jsc, and FF. The perovskite surface was modified with 4-bromobenzyl hydrobromide (BrBeAI) can improve the device's performance. |
|
General Description |
4-Bromobenzylamine (BBA), also known as p-bromobenzylamine, is an aryl bromide. The selective formation of nitrile or imine from BBA in the presence of red copper has been reported. The formal [4+4] reaction of BBA to form 2,6,9-triazabicyclo[3.3.1]nonane derivatives has been investigated. |
InChI:InChI=1/C7H8BrN/c8-7-3-1-6(5-9)2-4-7/h1-4H,5,9H2
The method was developed with the aim to determine 4-bromobenzylamine in the hydrogenation mixture after hydrogenation of 4-bromobenzonitrile. In the analysis of model mixtures the mean error of the determination was − 2.45% for the prim. and + 1.08% for the sec. amine.
Cobalt-doped hybrid materials consisting...
In this work, we exploit 4-bromobenzylamine hydriodate post-treatment on CsPbI2Br thin films to assist the extraction of holes and to block the flow of electrons to the hole transport layer through band engineering at the CsPbI2Br bulk/surface.
Herein, a Grubbs-catalyzed route for the...
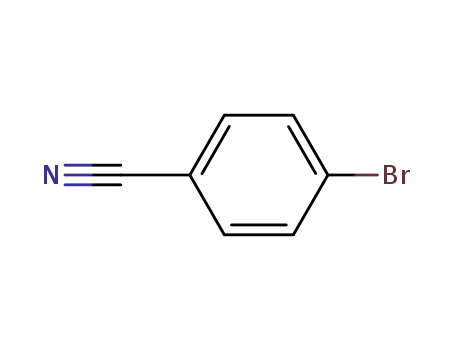
4-bromobenzenecarbonitrile

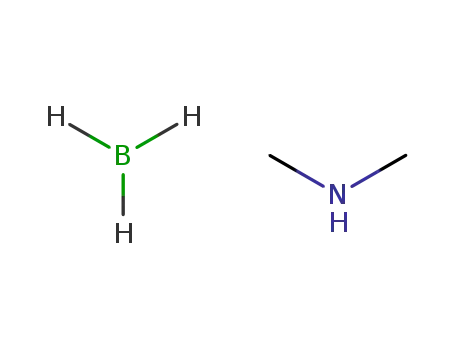
dimethylamine borane


4-Bromobenzylamine

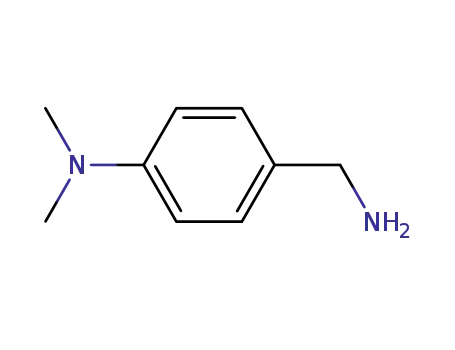
(4-aminomethylphenyl)dimethylamine

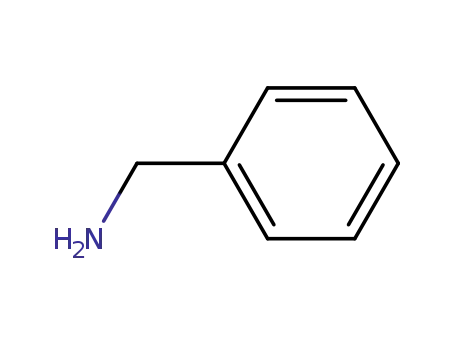
benzylamine
| Conditions | Yield |
|---|---|
|
dimethylamine borane; With n-butyllithium; In tetrahydrofuran; hexane; at 0 ℃;
4-bromobenzenecarbonitrile; In tetrahydrofuran; hexane; at 65 ℃; for 12h; Further stages.;
|
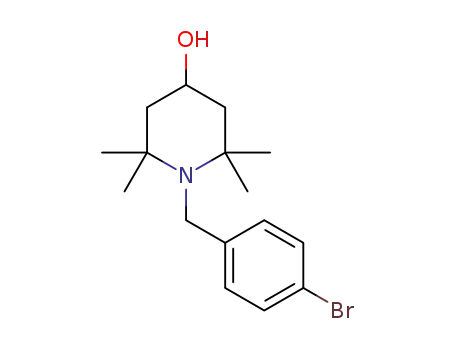
N-p-bromobenzyl-2,2,6,6-tetramethylpiperidin-4-ol

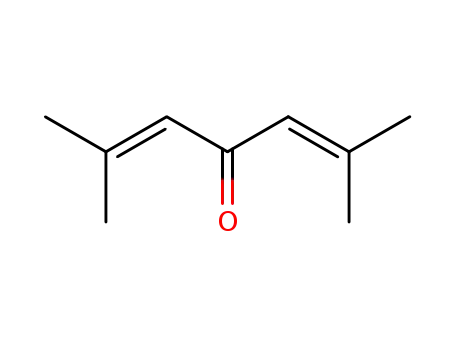
phorone


4-Bromobenzylamine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: C20H29ClO2P2Pt; 1-Phenylbut-1-en-3-one; sodium hydroxide / toluene / 4 h / 105 °C
2: hydrogenchloride / 5 h / 105 °C
With hydrogenchloride; C20H29ClO2P2Pt; 1-Phenylbut-1-en-3-one; sodium hydroxide; In toluene;
|
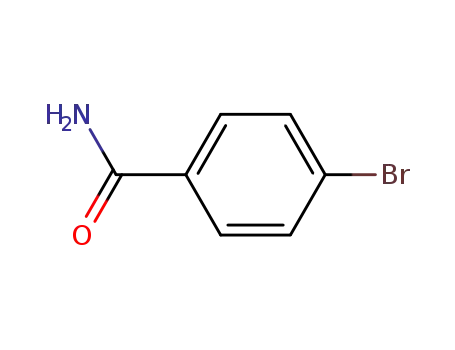
p-bromobenzamide
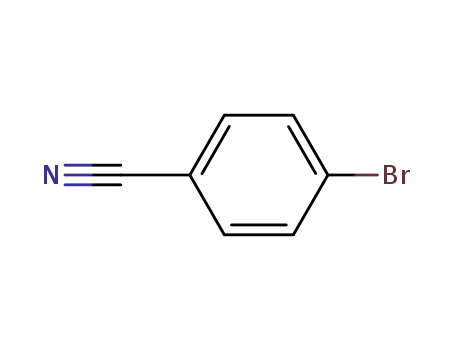
4-bromobenzenecarbonitrile
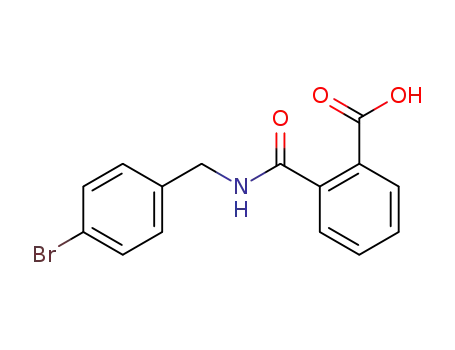
N-(4-bromo-benzyl)-phthalamic acid
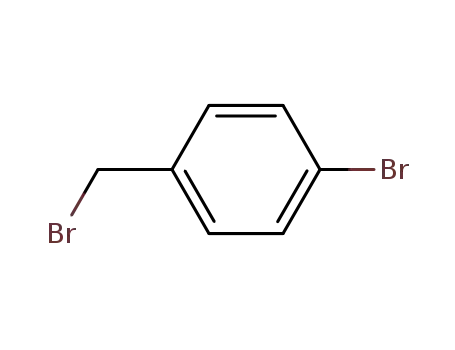
1-bromomethyl-4-bromobenzene
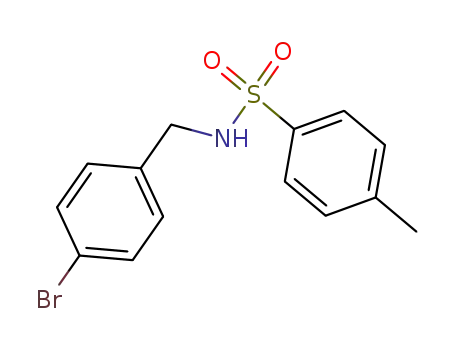
N-[(4-bromophenyl)methyl]-4-methylbenzenesulfonamide
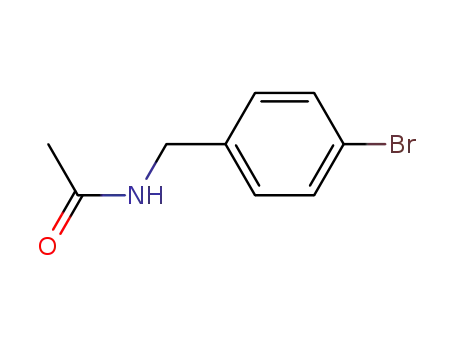
N-(4-bromo-benzyl)-acetamide
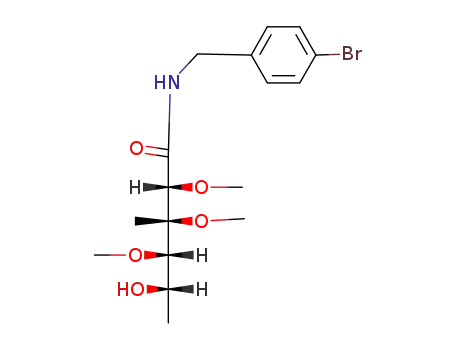
N-(p-Brombenzyl)nogalonamid
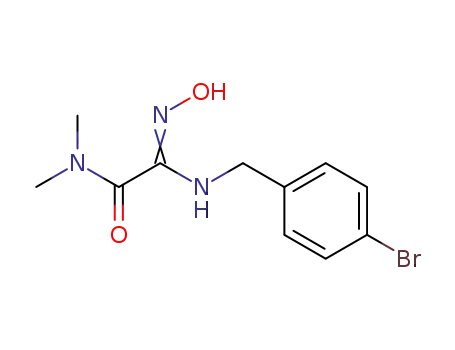
2-(4-Bromo-benzylamino)-2-[(Z)-hydroxyimino]-N,N-dimethyl-acetamide
CAS:1953-04-4
CAS:20826-04-4
CAS:137-00-8
CAS:10269-01-9