- Language:English
- English
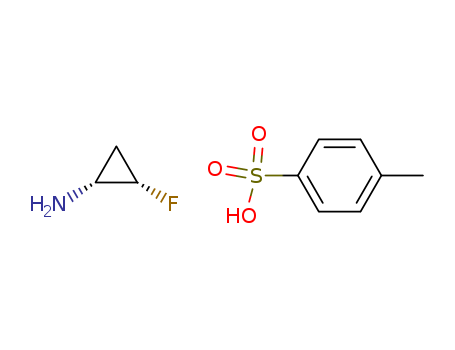

CasNo: 143062-84-4
Molecular Formula: C7H8O3S.C3H6FN
The invention discloses a preparation me...
A rhodium-catalyzed cyclopropanation of ...
cis-2-Fluorocyclopropylamine is stereose...
The title synthesis was achieved by empl...
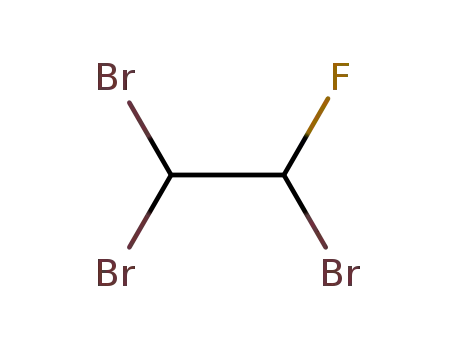
1,1,2-tribromo-2-fluoroethane

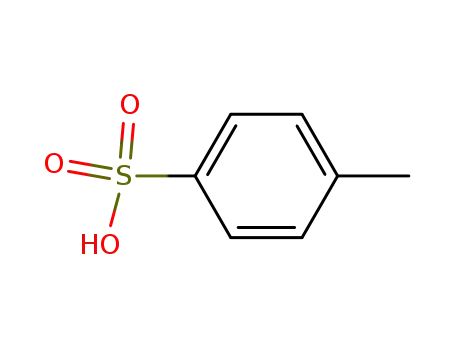
toluene-4-sulfonic acid

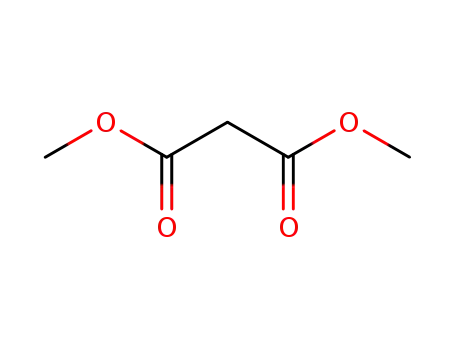
malonic acid dimethyl ester

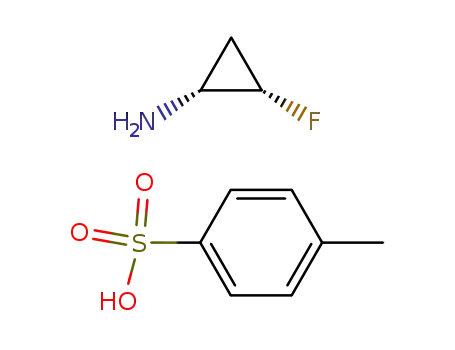
(1R,2S)-2-fluorocyclopropylamine p-toluenesulfonic acid salt
| Conditions | Yield |
|---|---|
|
1,1,2-tribromo-2-fluoroethane; malonic acid dimethyl ester;
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 25 ℃;
for 60h;
Large scale;
With
palladium on activated charcoal; hydrogen;
In
methanol;
for 24h;
under 3800.26 Torr;
Autoclave;
Large scale;
toluene-4-sulfonic acid;
Temperature;
Further stages;
Large scale;
|
204 g |

toluene-4-sulfonic acid

benzyl N-<(1R*,2S*)-2-fluorocyclopropyl>carbamate

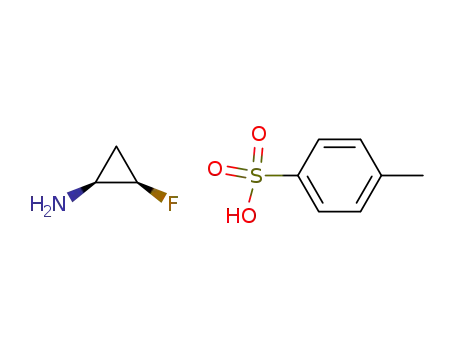
(1R*,2S*)-2-fluorocyclopropylammonium p-toluenesulfonate
| Conditions | Yield |
|---|---|
|
With
hydrogen;
palladium on activated charcoal;
In
methanol;
for 1.5h;
under 760 Torr;
Ambient temperature;
|
87% |

toluene-4-sulfonic acid
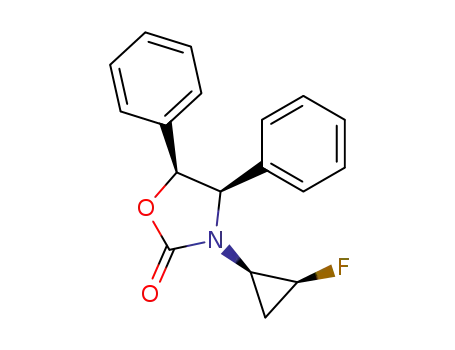
(4R,5S)-3-((1R,2S)-2-Fluoro-cyclopropyl)-4,5-diphenyl-oxazolidin-2-one
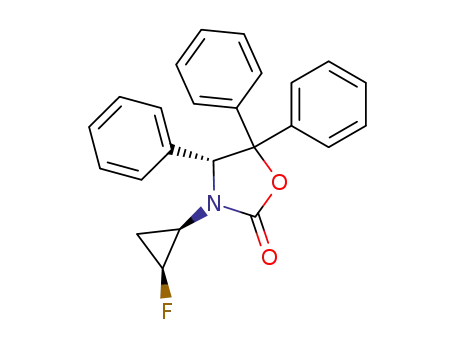
(R)-3-((1R,2S)-2-Fluoro-cyclopropyl)-4,5,5-triphenyl-oxazolidin-2-one
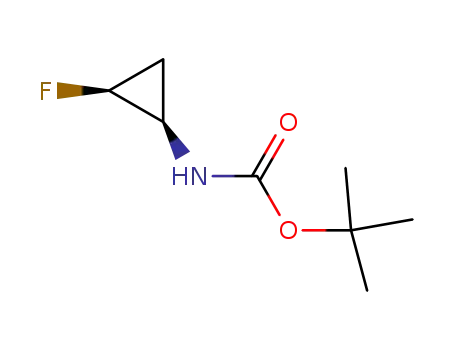
tert-butyl N-<(1R,2S)-2-fluorocyclopropyl>carbamate
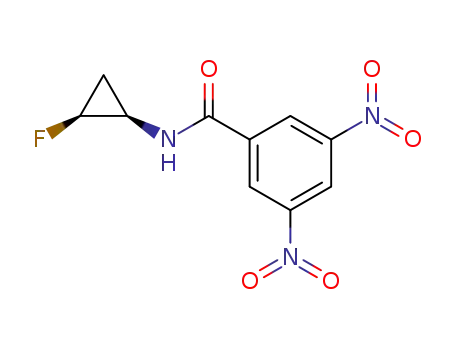
N-((1R,2S)-2-Fluoro-cyclopropyl)-3,5-dinitro-benzamide
CAS:84629-50-5
CAS:220127-57-1
CAS:1095-78-9
CAS:71675-87-1