- Language:English
- English
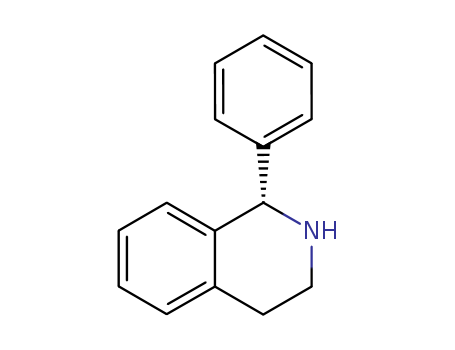

CasNo: 118864-75-8
Molecular Formula: C15H15N
Appearance: white solid
|
Chemical Properties |
White Solid |
|
Uses |
Labelled Solifenacin intermediate (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline is a type of organic compound that belongs to the class of tetrahydroisoquinolines. It is a chiral molecule with a single stereocenter at the 1-position. This compound has been found in a variety of natural products and has been the subject of extensive research due to its potential therapeutic properties. It has been reported to exhibit a range of pharmacological activities, including anticonvulsant, analgesic, and antidepressant effects. The structural simplicity and diverse biological activities of (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline make it an attractive target for synthetic and medicinal chemistry research. |
IUPAC Name: (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline
Isomeric SMILES: C1CN[C@H](C2=CC=CC=C21)C3=CC=CC=C3
InChIKey: PRTRSEDVLBBFJZ-HNNXBMFYSA-N
InChI: InChI=1S/C15H15N/c1-2-7-13(8-3-1)15-14-9-5-4-6-12(14)10-11-16-15/h1-9,15-16H,10-11H2/t15-/m0/s1
We report herein a simple alternative me...
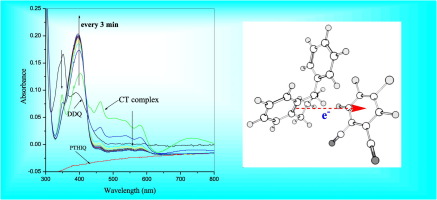
Molecular charge-transfer interaction of a series of electron π-acceptors of 1,4-benzoquinone (BQ), 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and Tetracyanoquinodimethane (TCNQ) with selected donors of 1-phenyl-1,2,3,4-tetrahydroisoquinoline (PTHIQ) and 4-aminoacetanilide (ACE) have been studied in methanol at room temperature. The stoichiometry of the complexes was determined by photometric titration method and was found to be 1:1, in all the cases.
The successful development of a catalyti...
Artificial metalloenzymes (ArMs) based o...
Tetrahydroisoquinolines (THIQs) with a C...
S(-)1-Phenyl-1,2,3,4-tetrahydro isoquino...
The title compounds (S)-(+)-8 and (R)-(-...
1-phenyl-3,4-dihydroisoquinoline

(S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline

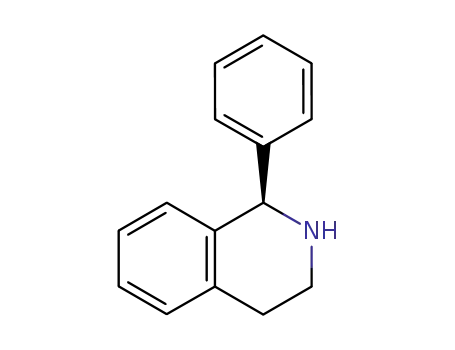
(R)-(-)-1-phenyl-1,2,3,4-tetrahydroisoquinoline
| Conditions | Yield |
|---|---|
|
With sodium tetrahydroborate; In ethanol; at 20 ℃; for 60h;
|
|
|
With hydrogen; bis(1,5-cyclooctadiene)diiridium(I) dichloride; (S)-(1,1'-binaphthalene)-2,2'-diylbis(diphenylphosphine); In tetrahydrofuran; 1,1-dichloroethane; at 20 - 50 ℃; under 22502.3 Torr; Product distribution / selectivity; Inert atmosphere;
|
50 % ee |
|
With 1,1'-bis-(diphenylphosphino)ferrocene; bis(1,5-cyclooctadiene)diiridium(I) dichloride; hydrogen; iodine; In dichloromethane; at 20 ℃; for 24h; under 38002.6 Torr; optical yield given as %ee; enantioselective reaction; Inert atmosphere; Autoclave;
|
|
|
With bis(1,5-cyclooctadiene)diiridium(I) dichloride; (R)-3,5-diMe-Synphos; hydrogen; In 1,4-dioxane; at 40 ℃; for 18h; under 22502.3 Torr; optical yield given as %ee; enantioselective reaction; Inert atmosphere;
|
|
|
With formic acid; [RuCl(η6-benzene)(1R,2R)-N-(naphthalene-1-sulfonyl)-1,2-diphenylethylenediamine]; triethylamine; In acetonitrile; at 30 ℃; Reagent/catalyst;
|
39 % ee |
|
With (2S,3R)-2-amino-3-hydroxybutanamide; streptavidin biotin-wild type; C42H64Cl4Ir2N6O4S2; In dimethyl sulfoxide; at 30 - 50 ℃; for 18h; pH=7.8; Reagent/catalyst; Overall yield = 96 %; enantioselective reaction;
|
63 % ee |
|
With C42H64Cl4Ir2N6O4S2; D-Phenylalaninamide; In dimethyl sulfoxide; at 30 - 50 ℃; for 18.25h; pH=7.8; Reagent/catalyst; Overall yield = 99 %; enantioselective reaction;
|
16 % ee |
|
With [N-[(1S,2S)-2-(amino-κN)-1,2-diphenylethyl]-4-methylbenzenesulfonamidato-κN]chloro[(1,2,3,4,5,6-η)-1,3,5-trimethylbenzene]ruthenium; formic acid; triethylamine; In acetonitrile; for 1.16667h; Optical yield = 2 %ee;
|
|
|
With [N-[(1S,2S)-2-(amino-κN)-1,2-diphenylethyl]-4-methylbenzenesulfonamidato-κN]chloro[(1,2,3,4,5,6-η)-1,3,5-trimethylbenzene]ruthenium; formic acid; triethylamine; In isopropyl alcohol; at 30 ℃; for 16h; Overall yield = 90 %; enantioselective reaction; Inert atmosphere;
|
29 % ee |
|
With N-[(1S,2S)-1,2-diphenyl-2-(3-phenylpropylamino)ethyl]-4-methylbenzene sulfonamide ammonium chloride ruthenium; hydrogen; trifluoroacetic acid; In methanol; at 40 ℃; for 6h; under 11251.1 Torr; Reagent/catalyst; Autoclave; Sealed tube;
|
11 % ee |
|
With hydrogen; In tetrahydrofuran; toluene; at 30 ℃; for 17h; under 45603.1 Torr; Reagent/catalyst; Solvent; enantioselective reaction; Autoclave;
|
24 % ee |
|
With (pentamethylcyclopentadienyl)IrCl[κ2(N,N')-(S,S)-p-toluenesulfonylNCHPhCHPhNH2]; hydrogen; trifluoroacetic acid; In methanol; for 6h; under 11251.1 Torr; stereoselective reaction;
|
71 % ee |
|
With D-glucose; BmGDH; imine reductase from Stackebrandtia nassauensis; NADP; In aq. phosphate buffer; dimethyl sulfoxide; at 30 ℃; for 12h; pH=7; Overall yield = 81 %; Overall yield = 170 mg; enantioselective reaction; Enzymatic reaction;
|
51 % ee |
|
With formic acid; (1,2,3,4,5-pentamethylcyclopentadienyl)Ir[κ2(N,N')-CH3C6H4SO2NCHPhCHPhNH]; phosphoric acid; triethylamine; In isopropyl alcohol; at 30 ℃; for 3h; Reagent/catalyst; Solvent; Overall yield = 90 %; Overall yield = 28.4 mg; enantioselective reaction; Catalytic behavior; Inert atmosphere; Schlenk technique;
|
86 % ee |
|
With D-Glucose; NADPH; at 30 ℃; for 24h; enantioselective reaction; Enzymatic reaction;
|
30 % ee |
|
Multi-step reaction with 2 steps
1: borane-ammonia complex
2: LG-I-D11 / Resolution of racemate; Enzymatic reaction
With borane-ammonia complex; LG-I-D11;
|
|
|
With N-Bromosuccinimide; bis(1,5-cyclooctadiene)diiridium(I) dichloride; hydrogen; (R)-2,2'-bis(diphenylphosphanyl)-1,1'-binaphthyl; In 1,2-dichloro-ethane; at 0 ℃; for 36h; under 25858.1 Torr; Reagent/catalyst; Solvent; Overall yield = 92 %; Overall yield = 57 mg; enantioselective reaction; Catalytic behavior; Glovebox;
|
86 % ee |
|
With Cp*Ir(biot-p-L)Cl; streptavidin S112A-N118P-K121A mutant; In aq. buffer; at 37 ℃; for 4h; enantioselective reaction; Catalytic behavior; Sealed tube; Enzymatic reaction;
|
85 % ee |
|
With Cp*Ir(biot-p-L)Cl; streptavidin S112A-N118P-K121A mutant; In aq. buffer; at 37 ℃; for 4h; Reagent/catalyst; enantioselective reaction; Catalytic behavior; Sealed tube; Enzymatic reaction;
|
86 % ee |
|
With N-Bromosuccinimide; chloro(1,5-cyclooctadiene)rhodium(I) dimer; hydrogen; sodium carbonate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; In 1,2-dichloro-ethane; at 30 ℃; for 24h; under 25858.1 Torr; enantioselective reaction; Glovebox;
|
88 % ee |
|
With N-Bromosuccinimide; chloro(1,5-cyclooctadiene)rhodium(I) dimer; hydrogen; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; In 1,2-dichloro-ethane; at 0 ℃; for 36h; under 25858.1 Torr; Temperature; Reagent/catalyst; Solvent; Overall yield = 92 %; enantioselective reaction; Glovebox;
|
82 % ee |
|
With Cp*Ir(biot-p-L)Cl; streptavidin S112A-N118P-K121A mutant; sodium formate; In aq. buffer; at 37 ℃; for 48h; pH=6; Reagent/catalyst; Temperature; enantioselective reaction; Catalytic behavior;
|
86 % ee |
|
With Cp*Ir(biot-p-L)Cl; streptavidin S112R-N118P-K121A-S122M-L124Y mutant; sodium formate; In aq. buffer; at 37 ℃; for 48h; pH=6; Reagent/catalyst; Temperature; enantioselective reaction; Catalytic behavior;
|
78 % ee |
|
With sodium formate; sodium hydroxide; In aq. buffer; at 37 ℃; for 48h; pH=7; Reagent/catalyst; Catalytic behavior;
|
64 % ee |
|
With recombinant FPD-chimera streptavidin Sav-FPD; [Cp*Ir(biot-p-L)Cl]; In dimethyl sulfoxide; at 25 ℃; for 16h; pH=7; Reagent/catalyst; enantioselective reaction; Catalytic behavior; Enzymatic reaction;
|
60 % ee |
|
With bis(1,5-cyclooctadiene)diiridium(I) dichloride; C42H46FeP2; hydrogen; trifluoroacetic acid; In tetrahydrofuran; at 30 ℃; for 12h; under 38002.6 Torr; Reagent/catalyst; Temperature; Solvent; enantioselective reaction; Catalytic behavior; Autoclave; Schlenk technique;
|
85 % ee |
|
With Cp*Ir(biot-p-L)Cl; MASMTGGQQMGRDQAGITGTWYNQLGSTFIVTAGADGALTGTYESAVGNAESRYVLTGRYDSAPATDGSGTALGWTVAWKNNYRNAHSATTWSGQYVGGAEARINTQWLLTAGTTEANAWASTLVGHDTFTKVKPSAASIDAAKKAGVNNGNPLDAVQQGSGGGNGGGNGGGNGGGNIDGRGGGNASMTGGQQMGRDQAGITGTWYNQLGSTFIVTAGADGALTGTYVTARGNAESRYVLTGRYDSAPATDGSGTALGWTVAWKNNYRNAHSATTWSGQYVGGAEARINTQWLLTAGTTEANAWASTLVGCDTFTKVKPSAASIDAAKKAGVNNGNPLDAVQQ; In dimethyl sulfoxide; at 25 ℃; for 24h; pH=7; Reagent/catalyst; Sealed tube;
|
54 % ee |
|
With Cp*Ir(biot-p-L)Cl; MASMTGGQQMGRDQAGITGTWYNQLGSTFIVTAGADGALTGTYESAVGNAESRYVLTGRYDSAPATDGSGTALGWTVAWKNNYRNAHSATTWSGQYVGGAEARINTQWLLTAGTTEANAWASTLVGHDTFTKVKPSAASIDAAKKAGVNNGNPLDAVQQGSGGGNGGGNGGGNGGGNIDGRGGGNASMTGGQQMGRDQAGITGTWYNQLGSTFIVTAGADGALTGTYVTARGNAESRYVLTGRYDSAPATDGSGTALGWTVAWKNNYRNAHSATTWSGQYVGGAEARINTQWLLTAGTTEANAWKSTLVGCDTFTKVKPSAASIDAAKKAGVNNGNPLDAVQQ; In dimethyl sulfoxide; at 25 ℃; for 24h; pH=7; Reagent/catalyst; Sealed tube;
|
17 % ee |
|
With 1-dodecyl-3-methylimidazolium bis(2,4,4-trimethylpentyl)phosphinate; C31H33ClN2O8RuS3(2-)*2Na(1+); sodium formate; In n-heptane; water; at 60 ℃; Overall yield = 10 percentChromat.; enantioselective reaction; Inert atmosphere;
|
26 % ee |
|
With formic acid; C33H37ClN2O4RuS; triethylamine; In dichloromethane; at 20 ℃; for 96h; Reagent/catalyst; enantioselective reaction; Inert atmosphere; Schlenk technique;
|
49 % ee |
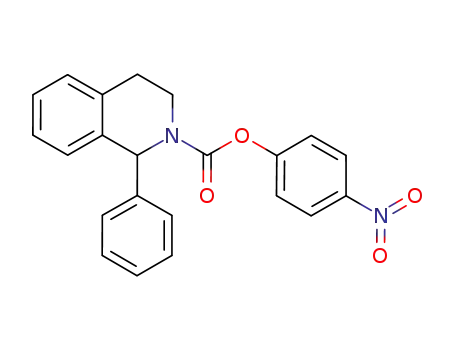
4-nitrophenyl-1-phenyl-3,4-dihydroisoquinoline-2(1H)-carboxylate


(S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline


(R)-(-)-1-phenyl-1,2,3,4-tetrahydroisoquinoline
| Conditions | Yield |
|---|---|
|
4-nitrophenyl-1-phenyl-3,4-dihydroisoquinoline-2(1H)-carboxylate; With sodium hydroxide; In ethanol; at 75 - 80 ℃;
With water; Cooling;
|
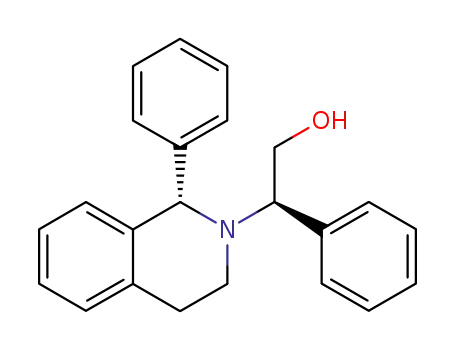
(1S,1'R)-2-(2-hydroxy-1-phenylethyl)-1-phenyl-1,2,3,4-tetrahydroisoquinoline
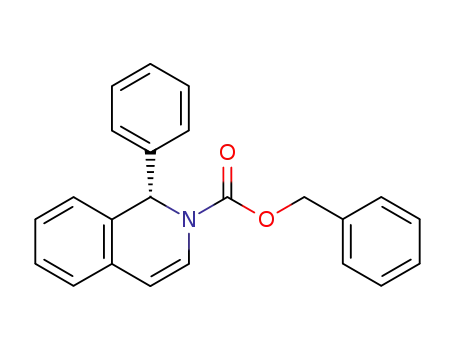
(S)-(-)-benzyl 1-phenyl-1,2-dihydroisoquinoline-2-carboxylate
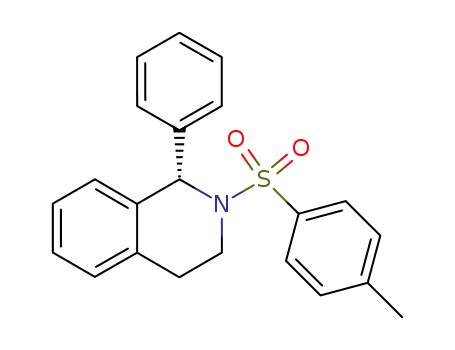
(S)-(-)-2-(4-methylphenylsulfonyl)-1-phenyl-1,2,3,4-tetrahydroisoquinoline
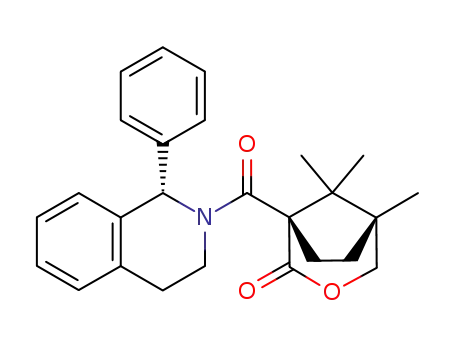
(1S,5R)-5,8,8-trimethyl-1-[(1S)-1-phenyl-1,2,3,4-tetrahydroisoquinolin-2-ylcarbonyl]-3-oxabicyclo[3.2.1]octan-2-one
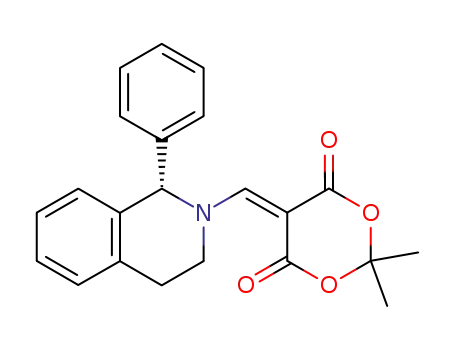
5-<2-(1-phenyl)tetrahydroisoquinolyl>methylene-2,2-dimethyl-1,3-dioxane-4,6-dione
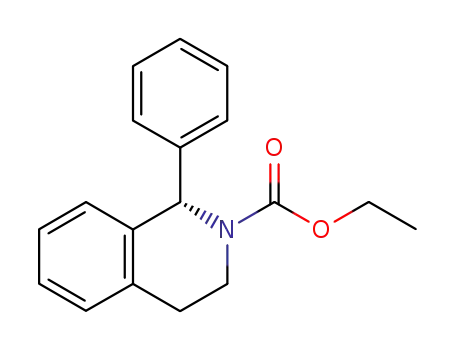
(S)-N-carboxylic acid ethyl ester-1-phenyl-1,2,3,4-tetrahydroisoquinoline
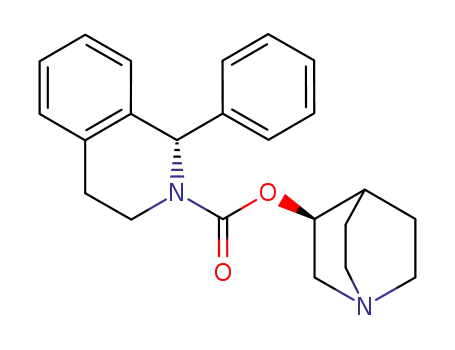
(1S)-3,4-dihydro-1-phenyl-2-(1H)-isoquinolinecarboxylic acid (3S)-1-azabicyclo[2.2.2]oct-3-yl ester
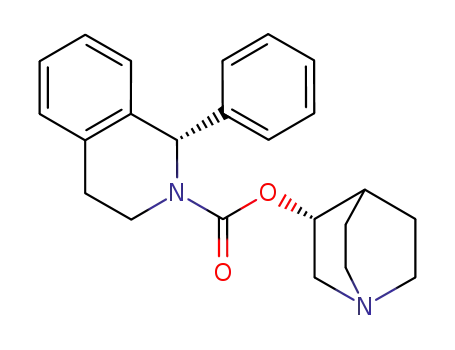
solifenacin
CAS:138071-82-6
CAS:38083-17-9
CAS:180272-14-4
CAS:146939-27-7