- Language:English
- English
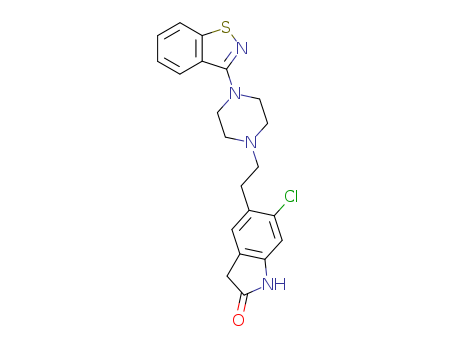

CasNo: 146939-27-7
Molecular Formula: C21H21ClN4OS
Appearance: tan solid
|
Atypical antipsychotic |
Ziprasidone is an atypical antipsychotic and belongs to piperazine benzothiazole compound. For in vitro condition, the product has a very high affinity to the dopamine D2, D3, 5-HT2A, 5-HT2C, 5-HT1A, 5-HT1D and α1 adrenergic receptor while having a moderate affinity to the histamine H1 receptor. It includes clozapine, olanzapine, quetiapine and ziprasidone. In vitro release profile of optimized ziprasidone loaded lipid phase-transition system (ZP-LPS) and ZP suspension in PBS release media containing 1% Tween 20 (n=3). |
|
Dosage and usage |
The initial dose is 2 times per day with 20mg each time through oral administration. The terminal T1/2 is about 7h. |
|
Uses |
Ziprasidone (ZP) is a novel atypical antipsychotic agent effective in the treatment of positive and negative symptoms of schizophrenia with low chances for extrapyramidal side effects (EPs) and cognitive deficits. It is a kind of antipsychotic and can be used as the antagonist of the dopamine D2-serotonin 5-HT2. |
|
Production method |
6-Chloro-1,3-dihydro-2H-indol-2-ketone (I) and bromoacetic acid can have acylation reaction under the action of polyphosphoric acid to give the compound (II). And then be stirred together with silicon hydride and triethyl trifluoroacetic acid at room temperature to give the compound (III). Finally, it can have reaction with N-(3-benzisothiazole-yl) piperazine in MIBK containing sodium carbonate to give the product. |
|
Chemical Properties |
Tan Solid |
|
Originator |
Geodon,Pfizer,USA |
|
Definition |
ChEBI: A piperazine compound having 1,2-benzothiazol-3-yl- and 2-(6-chloro-1,3-dihydro-2-oxindol-5-yl)ethyl substituents attached to the nitrogen atoms. |
|
Brand name |
Geodon (Pfizer). |
|
Therapeutic Function |
H-Indol-2-one, 5-(2-(4-(1,2-benzisothiazol-3-yl)-1- piperazinyl)ethyl)-6-chloro-1,3-dihydro-, monohydrochloride monohydrate |
|
Biological Functions |
"Ziprasidone is chemically similar to risperidone but with a substitution of piperzinyl and benzisothiazole for piperidinyl and benzisoxazole and with minor aromatic modification. Like risperidone, ziprasidone is a high-affinity antagonist at 5-HT2A/C and D2 receptors as well as at adrenergic α1/α2 and histamine H1 receptors. Moreover, ziprasidone can activate 5-HT1A receptors that regulate dopaminergic neurotransmission in brain regions involved in critical cognitive functions. Thus, in addition to D2 partial agonism, 5-HT1A agonism is now thought to be an important pharmacological property for atypical antipsychotic drug efficacy. |
|
General Description |
Ziprasidone (Geodon, a benzisothiazolpiprazinylindolonederivative) also has the structuralfeatures of a hybrid molecule between a butyrophenone antipsychoticand a trazodone-like antidepressant. It is highlymetabolized to four major metabolites, only one of which, Smethyldihydroziprasidone,likely contributes to its clinical activity. In humans, less than 5% of the dose isexcreted unchanged. Reduction by aldehyde oxidase accountsfor about 66% of ziprasidone metabolism; two oxidative pathwaysinvolving hepatic CYP3A4 account for the remainder. |
|
Mechanism of action |
Ziprasidone (half-life, 6 hours) has an oral bioavailability of approximately 60%, which can be enhanced in the presence of fatty foods. It is extensively metabolized (<5% excreted unchanged) by aldehyde oxidase, which results in reductive cleavage of the S–N bond, and then by S-methylation. Ziprasidone also can undergo CYP3A4-catalyzed N-dealkylation and S-oxidation. |
InChI:InChI=1/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27)/i7D2,8D2,9D2,10D2
In this study, Ziprasidone hydrochloride monohydrate (ZHM), the atypical drug used for SCZ treatment, was chosen as a model drug which belongs to the BCS Class II drugs that has low water solubility and high permeability.
Ziprasidone can induce glutamine metabolism disorder and redox state imbalance of PDAC cells by targeting GOT1, thereby inhibiting proliferation, preventing migration, and inducing apoptosis. Ziprasidone repressed glutamine metabolism and inhibited the growth of tumor in vivo.
A process for the preparation of oxindol...
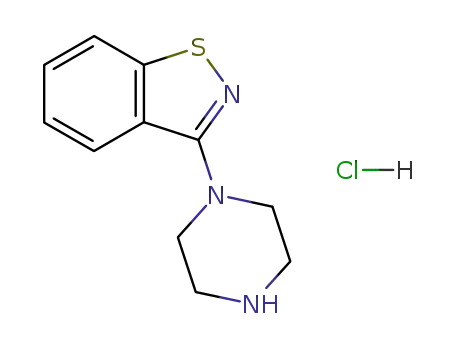
3-(1-piperazinyl)-1,2-benzisothiazole hydrochloride

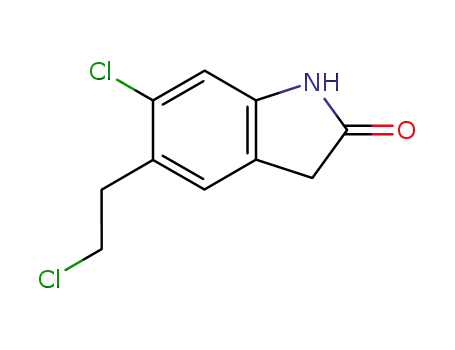
6-chloro-5-(2-chloroethyl)-2-oxindole

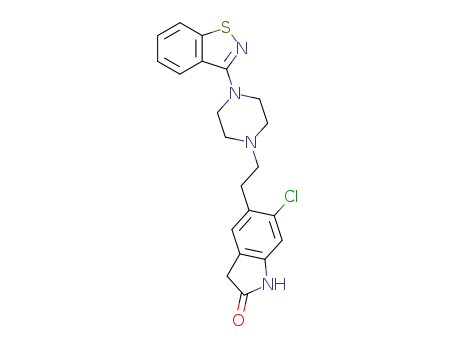
ziprazidone
| Conditions | Yield |
|---|---|
|
With sodium carbonate; In 1-methyl-pyrrolidin-2-one; at 130 - 135 ℃; for 24h; Product distribution / selectivity;
|
88.2% |
|
With sodium carbonate; In poly(ethylene glycol) methyl ether; at 120 - 125 ℃; for 48h; Product distribution / selectivity;
|
88% |
|
With sodium carbonate; sodium iodide; In toluene; for 22.5h; Product distribution / selectivity; Heating / reflux;
|
86% |
|
With sodium carbonate; In glycerol; at 115 ℃; for 6h; Product distribution / selectivity;
|
82.4% |
|
With sodium carbonate; sodium iodide; In sulfolane; at 90 ℃; for 4.5h; Product distribution / selectivity;
|
70% |
|
With N-ethyl-N,N-diisopropylamine; sodium iodide; In acetonitrile; at 121 - 122 ℃; for 25h; under 1500.15 Torr; Product distribution / selectivity;
|
56% |
|
With sodium carbonate; sodium iodide; In acetonitrile; at 80 - 125 ℃; for 25h; under 3750.38 Torr; Product distribution / selectivity;
|
51% |
|
With sodium carbonate; sodium iodide; In acetonitrile; at 120 - 125 ℃; for 25.1667h; under 3000.3 - 3750.38 Torr;
|
51% |
|
With sodium carbonate; In water; at 95 - 100 ℃; for 15h;
|
|
|
With sodium carbonate; sodium iodide; In butan-1-ol; at 100 - 110 ℃; for 8.5h; Product distribution / selectivity;
|
|
|
With sodium carbonate; sodium sulfate; In water; at 100 ℃; for 9 - 12h; Product distribution / selectivity; Heating / reflux;
|
|
|
With sodium carbonate; In water; butan-1-ol; for 20 - 35h; Product distribution / selectivity; Heating / reflux;
|
|
|
With sodium carbonate; at 90 - 95 ℃; for 10 - 16h; Product distribution / selectivity;
|
|
|
3-(1-piperazinyl)-1,2-benzisothiazole hydrochloride; With sodium carbonate; In glycerol; for 0.166667h;
6-chloro-5-(2-chloroethyl)-2-oxindole; In glycerol; at 115 - 120 ℃; for 3h; Product distribution / selectivity;
|
|
|
3-(1-piperazinyl)-1,2-benzisothiazole hydrochloride; 6-chloro-5-(2-chloroethyl)-2-oxindole; With sodium carbonate; In water; at 100 - 110 ℃;
With acetic acid; pH=6 - 7.5;
|
|
|
With sodium carbonate; In water; at 25 - 100 ℃;
|
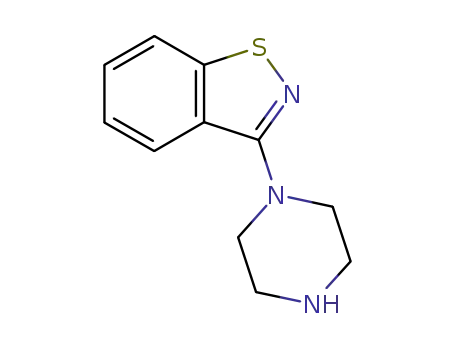
3-(1-piperazinyl)-1,2-benzisothiazole


6-chloro-5-(2-chloroethyl)-2-oxindole


ziprazidone
| Conditions | Yield |
|---|---|
|
With Morwet D425; In water; Reflux; Inert atmosphere;
|
92% |
|
In water; Inert atmosphere; Reflux; dispersing agent;
|
92% |
|
With potassium carbonate; potassium iodide; In sulfolane; at 75 - 100 ℃; for 2h; Product distribution / selectivity;
|
75% |
|
With potassium carbonate; potassium iodide; at 90 ℃; Product distribution / selectivity;
|
75% |
|
With sodium carbonate; In water; dimethyl sulfoxide; at 95 - 100 ℃; for 6 - 12h;
|
72% |
|
With sodium carbonate; In water; dimethyl sulfoxide; at 95 - 100 ℃; for 6 - 12h; Product distribution / selectivity;
|
72% |
|
In water; at 30 - 100 ℃; for 11 - 16.5h; Product distribution / selectivity;
|
68% |
|
With sodium carbonate; sodium iodide; In i-Amyl alcohol; for 72h; Heating;
|
|
|
With tetrabutylammomium bromide; sodium carbonate; sodium iodide; In cyclohexane; Heating / reflux;
|
|
|
With sodium carbonate; tetrabutyl phosphonium bromide; sodium iodide; In cyclohexane; at 95 - 102 ℃; under 1838.93 Torr; Heating / reflux;
|
|
|
With sodium carbonate; In water; for 15h; Heating / reflux;
|
|
|
With sodium carbonate; sodium iodide; tetrabutylammomium bromide; In cyclohexane; acetone; Heating / reflux;
|
|
|
With sodium carbonate; sodium iodide; tetrabutyl phosphonium bromide; In cyclohexane; acetone; at 95 - 102 ℃; under 1838.93 Torr; Product distribution / selectivity;
|
|
|
In water;
|
|
|
With sodium carbonate; sodium iodide; In water; dimethyl sulfoxide; at 115 - 125 ℃; for 2.75h; Product distribution / selectivity;
|
|
|
With sodium carbonate; sodium iodide; for 40h; Heating / reflux;
|
|
|
With sodium carbonate; In 1-ethyl-3-methyl-1H-imidazolium methylsulfate; at 100 ℃; for 24h; Product distribution / selectivity;
|
|
|
With sodium carbonate; In 1-butyl-3-methylimidazolium Tetrafluoroborate; at 100 ℃; for 24h; Product distribution / selectivity;
|
|
|
With sodium carbonate; In 1-n-butyl-3-methylimidazolim bromide; at 100 ℃; for 24h; Product distribution / selectivity;
|
|
|
With sodium carbonate; In 1-ethyl-3-methylimidazol-3-ium ethyl sulfate; at 100 ℃; Product distribution / selectivity;
|
|
|
|
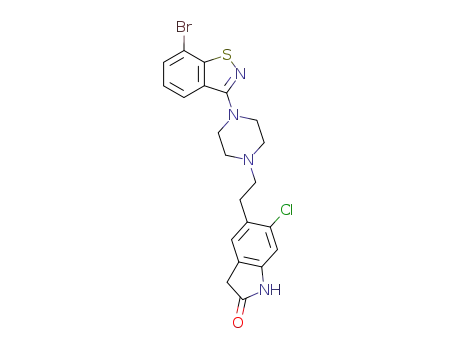
5-(2-(4-(7-bromo-1,2-benzisothiazol-3-yl)piperazinyl)-ethyl)-6-chloro-1,3-dihydro-2H-indol-2-one

3-(1-piperazinyl)-1,2-benzisothiazole

6-chloro-5-(2-chloroethyl)-2-oxindole
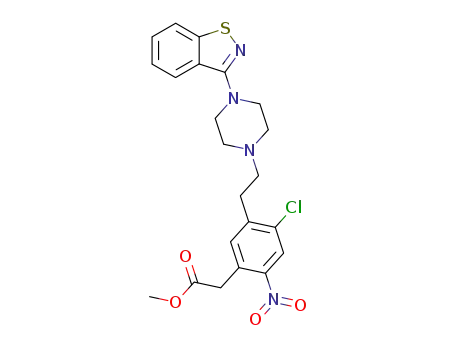
methyl 5-{2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl}-4-chloro-2-nitrophenyl acetate
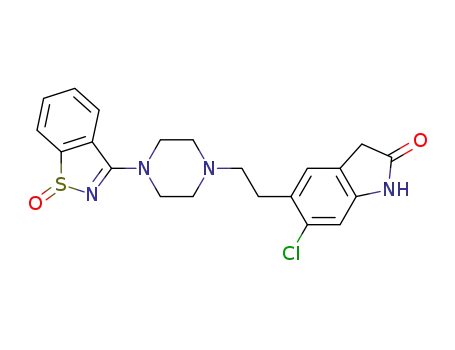
Ziprasidone sulfoxide
5-{2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-ethyl}-6-chloro-1,3-dihydro-2H-indol-2-one hydrobromide monohydrate
CAS:6038-19-3
CAS:51698-33-0
CAS:118864-75-8
CAS:20826-04-4