- Language:English
- English
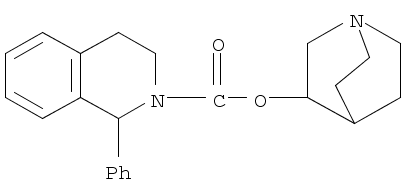

CasNo: 180272-14-4
Molecular Formula: C23H26N2O2
|
Uses |
Racemic Solifenacin Succinate is a muscarinic M3 receptor antagoinst used in treatment of urinary incontinence. |
IUPAC Name: 1-azabicyclo[2.2.2]octan-3-yl 1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate
Isomeric SMILES: C1CN2CCC1C(C2)OC(=O)N3CCC4=CC=CC=C4C3C5=CC=CC=C5
InChIKey: FBOUYBDGKBSUES-UHFFFAOYSA-N
InChI: InChI=1S/C23H26N2O2/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19/h1-9,18,21-22H,10-16H2
This study was a randomized placebo-controlled trial. There were 4 groups, group I received placebo, group II received tamsulosin 0.4 mg/day, group III received solifenacin 5 mg/day, and group IV received combination therapy of tamsulosin 0.4 mg/day added solifenacin 5 mg/day. Evaluation based on International Prostatic Symptom Score (IPSS) and Ureteral Stent Symptom Questioner (USSQ) score.
Two selective, sensitive, and green liquid chromatography methods were established and fully validated for quantitation of tamsulosin hydrochloride and solifenacin succinate along with four of their official and/or related impurities namely; tamsulosin sulfonic acid, tamsulosin impurity H, solifenacin impurity A and solifenacin impurity C.
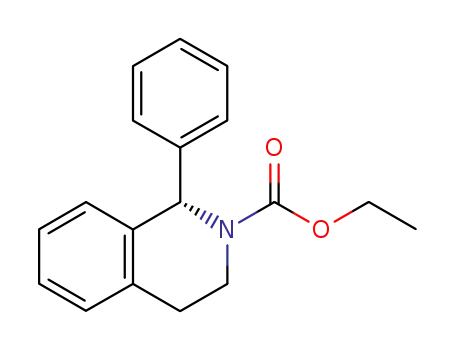
(S)-N-carboxylic acid ethyl ester-1-phenyl-1,2,3,4-tetrahydroisoquinoline

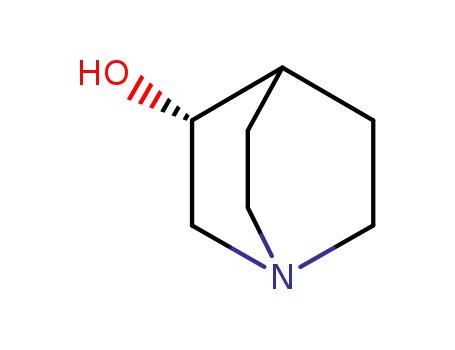
(R)-quinuclidin-3-ol

![(1S)-3,4-dihydro-1-phenyl-2-(1H)-isoquinolinecarboxylic acid (3S)-1-azabicyclo[2.2.2]oct-3-yl ester](/upload/2023/6/0816a24d-d92a-4631-bfcd-0a0b26f2d4ee.png)
(1S)-3,4-dihydro-1-phenyl-2-(1H)-isoquinolinecarboxylic acid (3S)-1-azabicyclo[2.2.2]oct-3-yl ester

![(3R)-1-azabicyclo[2.2.2]oct-3-yl (1R)-3,4-dihydro-1-phenyl-2(1H)-isoquinolinecarboxylate](/upload/2023/6/09b61e4c-a722-453f-b270-a4829eecb2d4.png)
(3R)-1-azabicyclo[2.2.2]oct-3-yl (1R)-3,4-dihydro-1-phenyl-2(1H)-isoquinolinecarboxylate

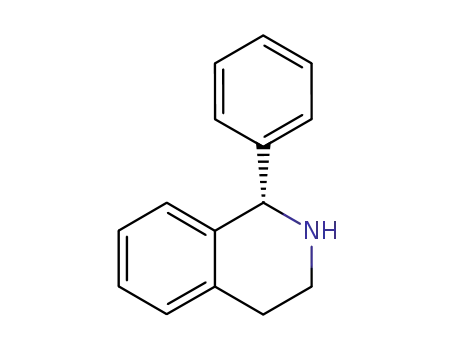
(S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline

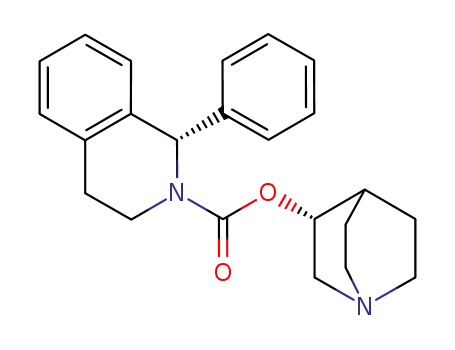
solifenacin
| Conditions | Yield |
|---|---|
|
With potassium 2-methylbutan-2-olate; In toluene; at 90 ℃; for 3h; Product distribution / selectivity;
|
1.1% 3.2% 6% |

(S)-N-carboxylic acid ethyl ester-1-phenyl-1,2,3,4-tetrahydroisoquinoline


(R)-quinuclidin-3-ol

![(1S)-3,4-dihydro-1-phenyl-2-(1H)-isoquinolinecarboxylic acid (3S)-1-azabicyclo[2.2.2]oct-3-yl ester](/upload/2023/6/0816a24d-d92a-4631-bfcd-0a0b26f2d4ee.png)
(1S)-3,4-dihydro-1-phenyl-2-(1H)-isoquinolinecarboxylic acid (3S)-1-azabicyclo[2.2.2]oct-3-yl ester

![(3R)-1-azabicyclo[2.2.2]oct-3-yl (1R)-3,4-dihydro-1-phenyl-2(1H)-isoquinolinecarboxylate](/upload/2023/6/09b61e4c-a722-453f-b270-a4829eecb2d4.png)
(3R)-1-azabicyclo[2.2.2]oct-3-yl (1R)-3,4-dihydro-1-phenyl-2(1H)-isoquinolinecarboxylate


(S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline


solifenacin
| Conditions | Yield |
|---|---|
|
With potassium 2-methylbutan-2-olate; In toluene; at 90 ℃; for 3h; Product distribution / selectivity;
|
1.1% 3.2% 6% |

(S)-N-carboxylic acid ethyl ester-1-phenyl-1,2,3,4-tetrahydroisoquinoline

(R)-quinuclidin-3-ol
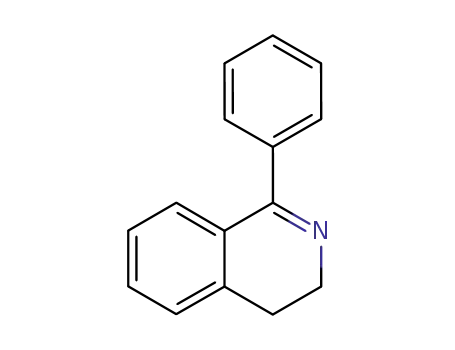
1-phenyl-3,4-dihydroisoquinoline
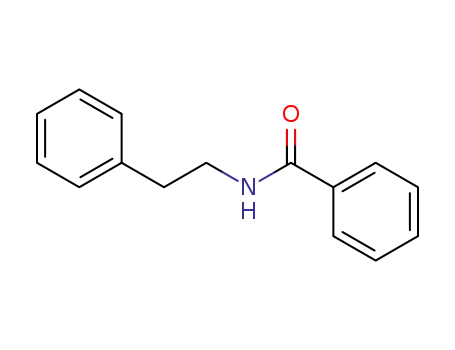
N-phenethylbenzamide
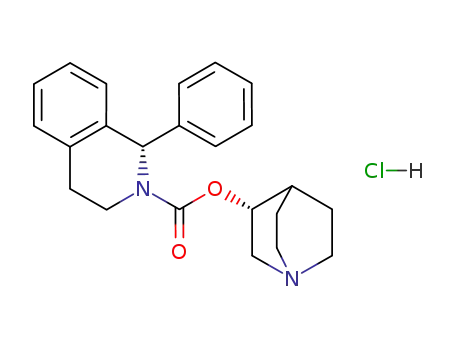
Solifenacin hydrochloride
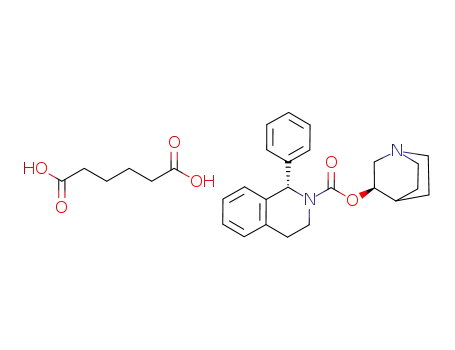
solifenacin hydrogen adipate

(1'S,3R)-3-[[(1'-phenyl-1',2',3',4'-tetrahydro-2'-isoquinolyl)carbonyl]oxy]quinuclidine 1-oxide
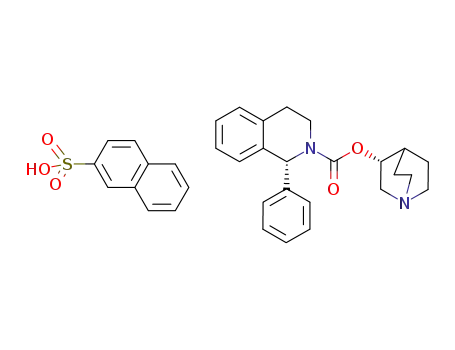
(-)-(3R)-quinuclidin-3-yl (1R)-1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylate naphthalene-2-sulfonate
CAS:138071-82-6
CAS:38083-17-9
CAS:1668-86-6
CAS:118864-75-8