- Language:English
- English
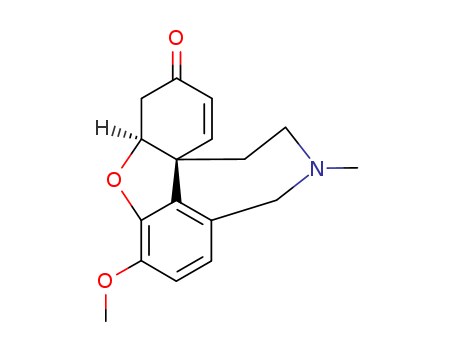

CasNo: 1668-86-6
Molecular Formula: C17H19NO3
|
Uses |
(+/-)-Narwedine is a natural product that has been studied for its potential therapeutic properties. It increases the amplitude and decreases the frequency of cardiac contractions and would therefore be of value in reducing blood loss during surgery. It also inhibits the action of narcotics and hypnotics, and increases the analgesic effect of morphine as well as the pharmacological effects of caffeine, carbazole, arecoline, and nicotine. There was another postulation that narwedine is the precursor for galanthamine production. |
IUPAC Name: (1S,12S)-9-methoxy-4-methyl-11-oxa-4-azatetracyclo[8.6.1.01,12.06,17]heptadeca-6(17),7,9,15-tetraen-14-one
Isomeric SMILES: CN1CC[C@@]23C=CC(=O)C[C@@H]2OC4=C(C=CC(=C34)C1)OC
InChIKey: QENVUHCAYXAROT-YOEHRIQHSA-N
InChI: InChI=1S/C17H19NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,14H,7-10H2,1-2H3/t14-,17-/m0/s1
The researchers synthesized a total of 16 narwedine derivatives and evaluated their anti-inflammatory activity in vitro. They found that some of the derivatives exhibited promising anti-inflammatory activity, indicating that they may have potential as therapeutic agents for the treatment of inflammatory diseases.
The total synthesis of (±)-galanthamine ...
The researchers evaluated the effects of (+/-)-narwedine on lipopolysaccharide (LPS)-induced nitric oxide (NO) production and tumor necrosis factor-alpha (TNF-alpha) release in macrophages in vitro, as well as its inhibitory effects on the growth of bacteria and fungi. They found that (+/-)-narwedine exhibited significant anti-inflammatory and anti-infectious effects, suggesting that it may have potential as a therapeutic agent for the treatment of inflammatory and infectious diseases.
Alkaloids including galanthamine (1) and...
C18H22BN2O4(1+)

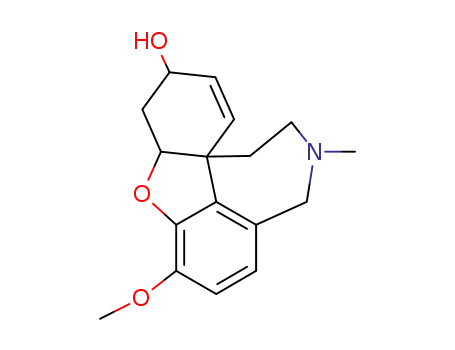
![(4aS,8aS)-rel-3-methoxy-11-methyl-4a,5,9,10,11,12-hexahydro-6H-benzo[2,3]benzofuro[4,3-cd]azepin-6-one](/upload/2023/6/c2e5e9d8-b1f5-49d2-bee9-56d8f52eae1b.png)
(4aS,8aS)-rel-3-methoxy-11-methyl-4a,5,9,10,11,12-hexahydro-6H-benzo[2,3]benzofuro[4,3-cd]azepin-6-one
| Conditions | Yield |
|---|---|
|
With methanesulfonic acid; In 1,4-dioxane; at 80 ℃; for 0.666667h;
|
74% |
galantamine

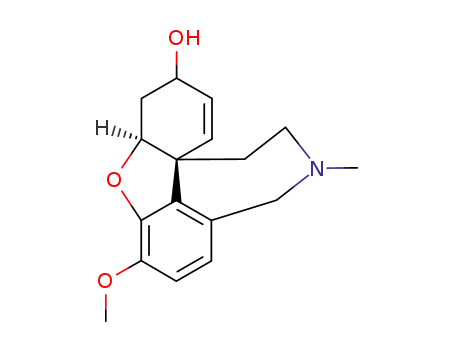
![(4aS,8aS)-rel-3-methoxy-11-methyl-4a,5,9,10,11,12-hexahydro-6H-benzo[2,3]benzofuro[4,3-cd]azepin-6-one](/upload/2023/6/c2e5e9d8-b1f5-49d2-bee9-56d8f52eae1b.png)
(4aS,8aS)-rel-3-methoxy-11-methyl-4a,5,9,10,11,12-hexahydro-6H-benzo[2,3]benzofuro[4,3-cd]azepin-6-one
| Conditions | Yield |
|---|---|
|
With manganese(IV) oxide; In acetone; at 25 - 30 ℃; for 20h;
|
60% |
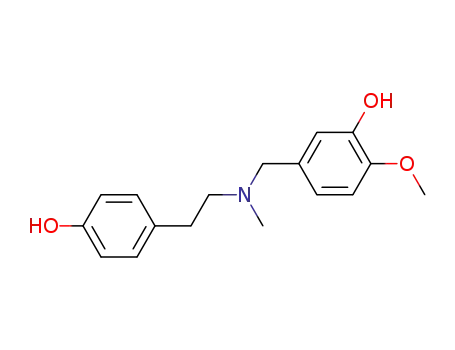
4'-O-methyl-N-methylnorbelladine
4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6H-benzofuro[3a,3,2-ef ][2]benzazepin-6-ol
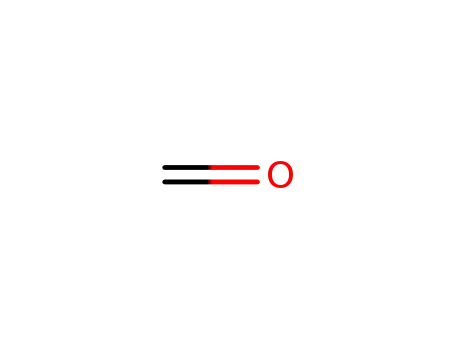
formaldehyd
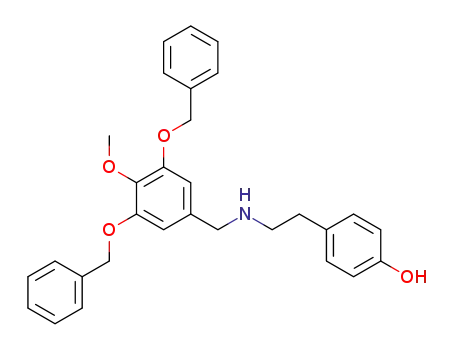
N-(3,5-dibenzyloxy-4-methoxy)benzyl-N-(4-hydroxyphenyl)ethylamine
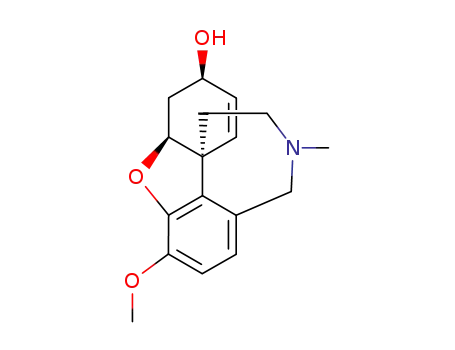
galanthamine
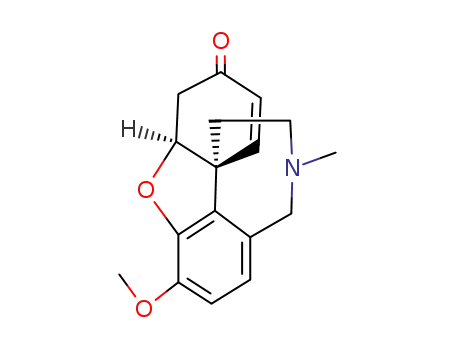
(-)-narwedine
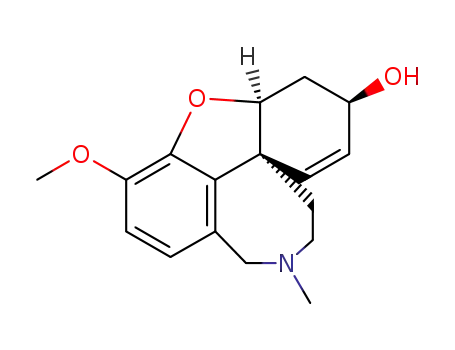
Galantamine
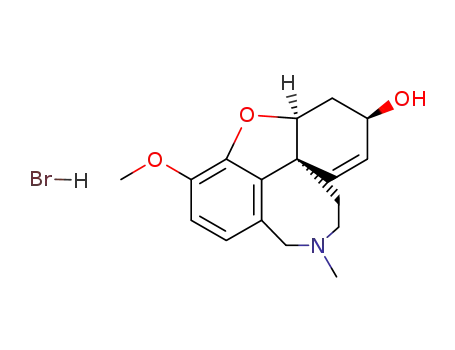
Galantamine hydrobromide
CAS:138071-82-6
CAS:38083-17-9
CAS:1953-04-4
CAS:180272-14-4