- Language:English
- English


CasNo: 1823-59-2
Molecular Formula: C16H6O7
Appearance: off-white to white powder
|
Description |
ODPA is a solid, melts in the range of 225-229 °C and was first introduced in the mid-1980s, offering a greater degree of flexibility than other commercial dianhydrides. It also is somewhat less reactive than BTDA.? ODPA has largely been used in polyimides and much less so in epoxies. It imparts special performance properties in free films and in FCCL production for high-end personal electronic devices. |
|
Chemical Properties |
off-white to white powder |
|
Uses |
4,4'-oxydiphthalic anhydride be used for the preparation of a polyimide material. |
|
Preparation |
The preparation of 4,4'-Oxydiphthalic anhydride is as follows:Chlorophthalic anhydride (30g, 160mmol), tetraphenyl phosphonium bromide (0.3g, 0.7mmol), and 2,5-dichlorobenzoic acid (0.08g, 0.4mmol) were heated to approximately 220℃. Potassium carbonate (7.95g, 58mmol) was added over 45 minutes. The reaction was sampled after addition of the potassium carbonate and showed a 24.9% conversion to 4,4'-oxydiphthalic anhydride. The reaction was heated for an additional 45 minutes. The reaction mixture was diluted with 1,2,4-trichlorobenzene (90g) and filtered. The 4,4'-oxydiphthalic anhydride was allowed to crystallize and was collected, washed with 1,2,4-trichlorobenzene followed by hexane, and dried in an air circulating oven at 120℃. to give crude 4,4'-oxydiphthalic anhydride, 12.9g. |
|
Flammability and Explosibility |
Notclassified |
InChI:InChI=1/C16H6O7/c17-13-9-3-1-7(5-11(9)15(19)22-13)21-8-2-4-10-12(6-8)16(20)23-14(10)18/h1-6H
The invention provides a preparation met...
The invention discloses a preparation me...
The invention relates to the technical f...
The refined product is subjected to, hea...
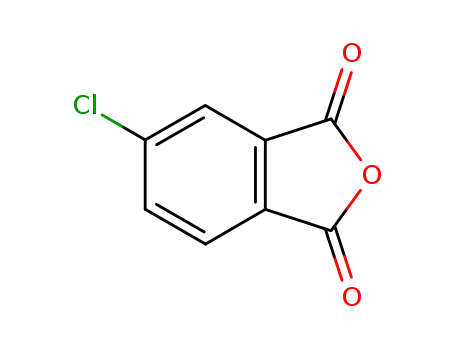
4-chlorophthalic anhydride

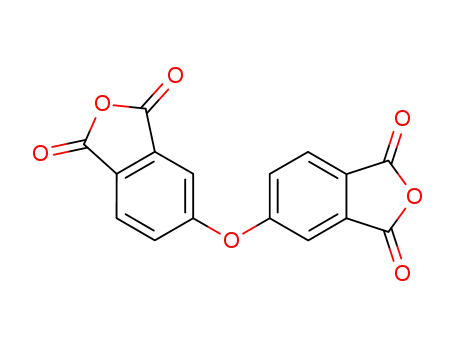
4,4'-oxydiphthalic dianhydride
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
hexaethylguanidinium chloride;
In
water; 1,2-dichloro-benzene;
for 3.5h;
Heating / reflux;
|
85% |
|
With
potassium carbonate; sodium nitrite;
In
N,N-dimethyl acetamide; toluene;
at 162 - 170 ℃;
for 5h;
|
85% |
|
4-chlorophthalic anhydride;
With
monopotassium carbonate;
tetraphenylphosphonium bromide; potassium hydrogencarbonate;
In
2,4-dichlorotoluene;
for 7.5h;
Heating / reflux;
Nitrogen atmosphere;
Conversion of starting material;
Heating / reflux;
|
82% |
|
With
monopotassium carbonate;
tetraphenylphosphonium bromide; potassium hydrogencarbonate;
In
1,2-dichloro-benzene;
Conversion of starting material;
Heating / reflux;
|
80% |
|
With
tetraphenylphosphonium bromide; potassium carbonate; caesium carbonate; 1,2,4-Trichlorobenzene;
Reagent/catalyst;
Inert atmosphere;
|
72% |
|
With
N-[bis(diethylamino)methylene]-N-ethylethane ammonium chloride; potassium carbonate;
In
1,2-dichloro-benzene;
for 8h;
|
|
|
With
potassium carbonate;
N-[bis(diethylamino)methylene]-N-ethylethane ammonium chloride;
In
dichlorobenzene, 1,2-;
at 210 ℃;
Product distribution / selectivity;
Molecular sieve;
Inert atmosphere;
|
|
|
With
potassium carbonate;
N-[bis(diethylamino)methylene]-N-ethylethane ammonium chloride;
In
dichlorobenzene, 1,2-;
at 210 ℃;
Product distribution / selectivity;
Molecular sieve;
Inert atmosphere;
|
|
|
With
potassium carbonate;
N-[bis(diethylamino)methylene]-N-ethylethane ammonium chloride;
In
1,2-dichloro-benzene;
at 210 ℃;
for 4h;
Inert atmosphere;
|
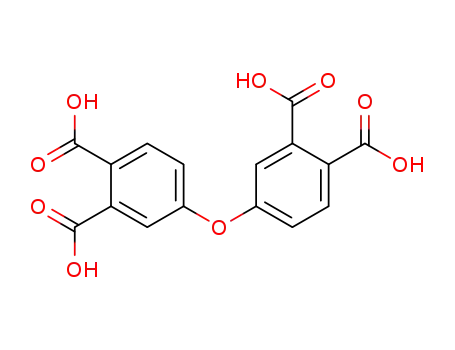
4,4'-oxydiphthalic acid


4,4'-oxydiphthalic dianhydride
| Conditions | Yield |
|---|---|
|
With
2,6-bis[(2,2,6,6-tetramethylpiperidin-1-yl)methyl]phenylboronic acid;
In
propyl cyanide;
for 12h;
Reflux;
|
90% |
|
In
water;
at 120 ℃;
Purification / work up;
|
|
|
In
1,2-dichloro-benzene;
at 200 ℃;
Product distribution / selectivity;
Heating / reflux;
|
|
|
at 120 - 220 ℃;
for 24h;
|
|
|
at 210 - 230 ℃;
|

4-chlorophthalic anhydride

4,4'-oxydiphthalic acid
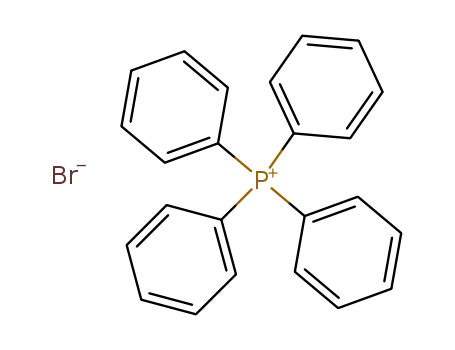
tetraphenylphosphonium bromide
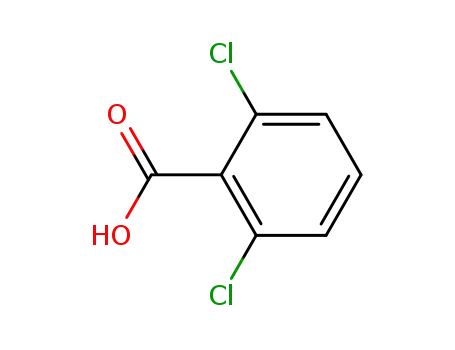
2,6-dichlorobenzoic acid

4,4'-oxydiphthalic acid
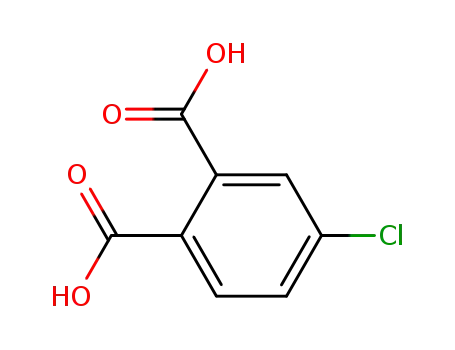
4-chlorophthalic acid
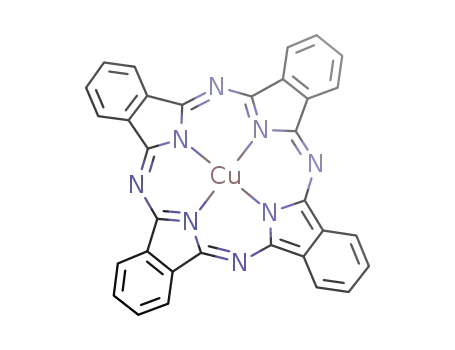
copper phthalocyanine
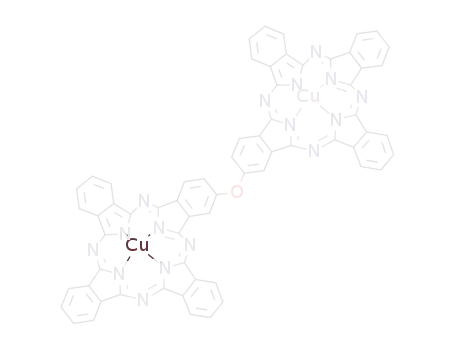
C64H30Cu2N16O
CAS:138071-82-6
CAS:57280-22-5
CAS:36978-41-3