- Language:English
- English
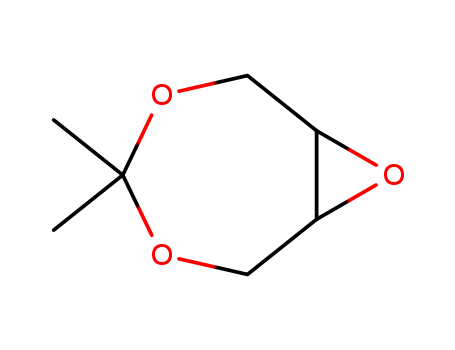

CasNo: 57280-22-5
Molecular Formula: C7H12O3
Enantioselective synthesis of the hexahy...
The invention discloses a method for eff...
The invention disclose a 4,4-dimethyl 3,...
The diastereoselective synthesis of fluo...
2,2-dimethyl-4,7-dihydro-1,3-dioxepin

4,4-dimethyl-3,5,8-trioxabicyclo[5.1.0]-octane
| Conditions | Yield |
|---|---|
|
With disodium hydrogenphosphate; dihydrogen peroxide; sodium hydroxide; In methanol; water; acetonitrile; at 60 - 80 ℃; pH=7 - 9.5; Large scale; Green chemistry;
|
85.2% |
|
With disodium hydrogenphosphate; 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 20 ℃; for 18h; Inert atmosphere;
|
85% |
|
With disodium hydrogenphosphate; dihydrogen peroxide; Hexafluoroacetone; In 1,2-dichloro-ethane; for 24h; Heating;
|
83% |
|
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane;
|
80% |
|
With sodium hydroxide; tert-butylhypochlorite; sodium hydrogensulfite; In aqueous t-butanol;
|
80% |
|
With dihydrogen peroxide; sodium carbonate; In methanol; benzonitrile;
|
70% |
|
With dihydrogen peroxide; sodium carbonate; In methanol; acetonitrile;
|
67% |
|
With disodium hydrogenphosphate; phosphomolybdic acid; dihydrogen peroxide; sodium hydroxide; In methanol; water; at 40 - 60 ℃; for 3h; pH=8 - 10; Reagent/catalyst;
|
|
|
Multi-step reaction with 2 steps
1: N-Bromosuccinimide; water / tetrahydrofuran / 2 h / 20 °C
2: potassium carbonate / methanol / 16 h / 20 °C
With N-Bromosuccinimide; water; potassium carbonate; In tetrahydrofuran; methanol;
|
C7H13BrO3

4,4-dimethyl-3,5,8-trioxabicyclo[5.1.0]-octane
| Conditions | Yield |
|---|---|
|
With potassium carbonate; In methanol; at 20 ℃; for 16h;
|
19 g |
2,2-dimethyl-4,7-dihydro-1,3-dioxepin
2,2-dimethoxy-propane
2-(3-Methoxy-2-methoxymethoxy-5-methyl-phenyl)-propan-2-ol
3-Hydroxy-4-(3-methoxy-2-methoxymethoxy-5-methyl-phenyl)-butan-2-one
4-Hydroxy-3-(3-methoxy-2-methoxymethoxy-5-methyl-phenyl)-butan-2-one
trans-6-benzylamino-2,2'-dimethyl-1,3-dioxocycloheptan-5-ol
CAS:138071-82-6
CAS:38083-17-9
CAS:3077-13-2
CAS:530-62-1