- Language:English
- English


CasNo: 36978-41-3
Molecular Formula: C16H6O6
|
Uses |
2,3,3',4'-Biphenyltetracarboxylic dianhydride (α-BPDA) is a chemical compound that belongs to the class of tetracarboxylic dianhydrides. It is also known by its chemical formula C16H6O6. This compound has been studied for its potential use in various applications such as in the field of organic electronics, as well as in medicinal chemistry for its potential therapeutic properties. 2,3,3',4'-Biphenyltetracarboxylic dianhydride be used for the preparation of a polyimide material. |
|
Preparation |
The preparation of 2,3,3',4'-biphenyl tetracarboxylic dianhydride is as follows:In a 1000 ml three-necked flask, 18.3 g (0.1 mol) of 4-chlorophthalic anhydride and 18.3 g (0.1 mol) were added 3-chlorophthalic anhydride, 300 g of anisole as solvent, 0.13 g (0.001 mol) of nickel chloride as a catalyst, 0.198 g (0.001 mol) of C-1 as a catalyst ligand, and 0.03 g (0.0003 mol) of sodium bromide as a catalyst Auxiliary, 13 g (0.2 mol) of zinc powder was used as a reducing agent, and the reaction was stirred at 30 ° C for 8 hours under a nitrogen atmosphere. The reaction solution was filtered to remove insoluble solids in the reaction liquid. 300 g of methanol was added to the filtration mother liquor, and the product was precipitated, filtered, and dried to obtain 25.8 g of the product 2,3,3',4'-biphenyltetracarboxylic dianhydride, yield 87.7%. |
Polyimides derived from 2,3,3',4'-BIPHENYL TETRACARBOXYLIC DIANHYDRIDE (α-BPDA) have higher Tg and transmittances, and possess lighter colored than the corresponding symmetric polyimides based on s-BPDA, but the improvement of solubility is limited.
The invention discloses a method for pre...
The invention discloses a method for pre...
In order to investigate the high-temperature performance of 2,3,3',4'-BIPHENYL TETRACARBOXYLIC DIANHYDRIDE (α-BPDA)-based polyimide composites, fiber-reinforced composites were prepared, and the high-temperature mechanical and thermophysical properties were tested.
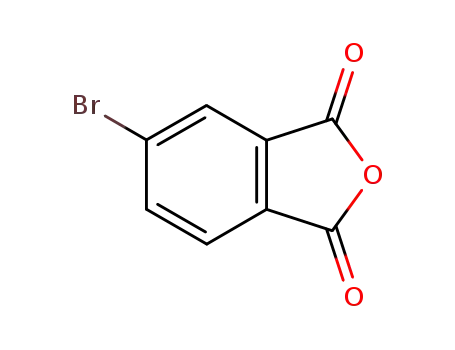
4-bromophthalic anhydride

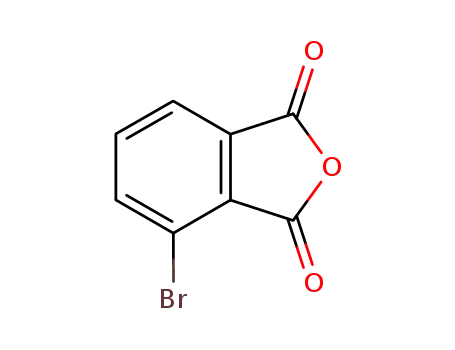
3-bromophthalic anhydride

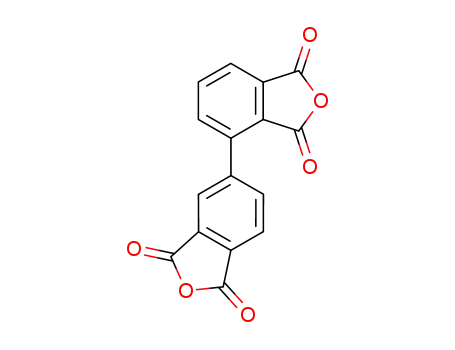
2,3',3,4'-biphenyltetracarboxylic acid dianhydride
| Conditions | Yield |
|---|---|
|
With N-phenylpicolinamide; sodium bromide; nickel dichloride; zinc; at 30 ℃; for 8h; Inert atmosphere;
|
76.19% |
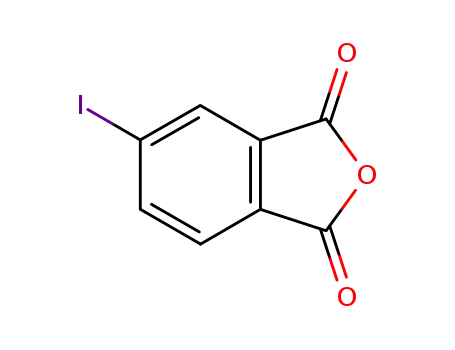
4-iodophthalic anhydride

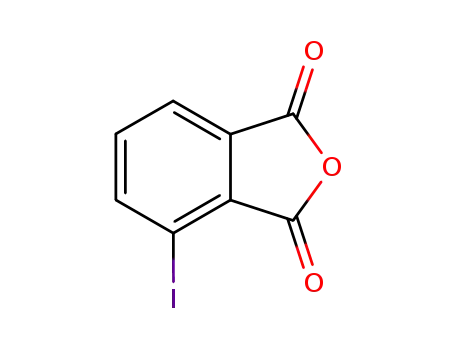
3-iodophthalic anhydride


2,3',3,4'-biphenyltetracarboxylic acid dianhydride
| Conditions | Yield |
|---|---|
|
With N-phenylpicolinamide; sodium bromide; nickel dichloride; zinc; at 30 ℃; for 8h; Inert atmosphere;
|
74.15% |
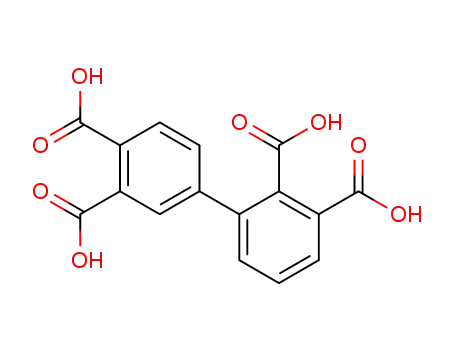
2,3,3′,4′-biphenyl tetracarboxylic dianhydride
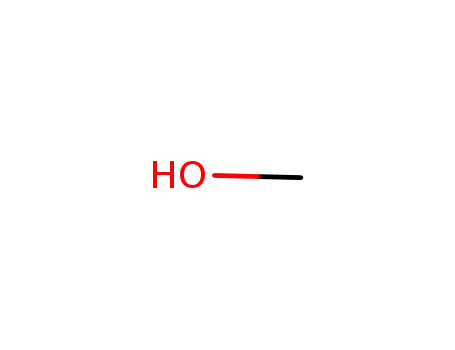
methanol
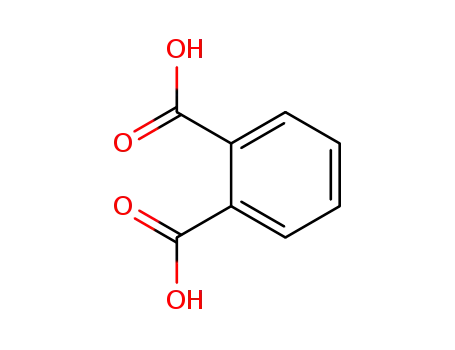
benzene-1,2-dicarboxylic acid

4-iodophthalic anhydride
CAS:138071-82-6
CAS:57280-22-5
CAS:2420-87-3
CAS:1823-59-2