- Language:English
- English
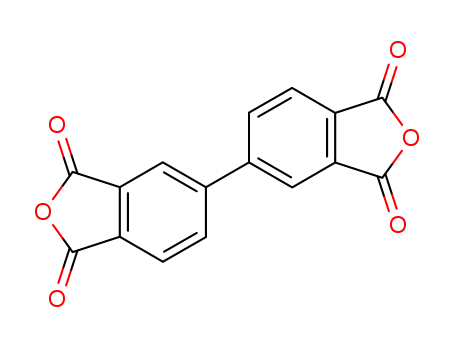

CasNo: 2420-87-3
Molecular Formula: C16H6O6
|
Chemical Properties |
Off-white solid |
|
Uses |
3,3',4,4'-biphenyltetracarboxylic dianhydride be used for the preparation of a polyimide material. This compound has been studied for its potential use in various applications such as in the field of organic electronics, as well as in medicinal chemistry for its potential therapeutic properties. |
|
Preparation |
The preparation of 3,3',4,4'-Biphenyltetracarboxylic dianhydride is as follows:In a nitrogen atmosphere, add N-(3-N,N-dimethylamino-propyl)-4-chlorophthalimide (26.65g, 0.1moL),Zinc powder (3.25g, 0.05moL), anhydrous NiCl2 (127.5mg, 1mmoL),Triphenylphosphine (250mg, 1mmoL) and 70 mL of anhydrous DMAc were stirred at 50°C for 24 hours, and 55mL of solvent DMAc was recovered under reduced pressure. Add 80g of xylene to the system and reflux (recrystallize), filter out the inorganic matter, the clarified filtrate will be cooled and precipitated, filtered, and vacuum dried for 10 hours. 21.8g of 3,3',4,4'-biphenylbisimine was obtained with a yield of 94%. In a 100mL reaction flask, add 4.62g (0.01mol) of the above 3,3',4,4'-biphenylbisimine and 9g of 20% sodium hydroxide aqueous solution, and heat to reflux for 24 hours. Filter and adjust pH=1 with concentrated hydrochloric acid to obtain 3,3’,4,4’-biphenyltetracarboxylic acid. After filtering, wash with water three times and reflux with water with 20mL trimethylbenzene.2.85g of white 3,3′,4,4′-biphenyltetracarboxylic dianhydride was obtained, the yield was 97%. |
|
General Description |
3,3',4,4'-Biphenyltetracarboxylic dianhydride (BPDA) is a chemical compound that belongs to the class of benzofuran derivatives. |
|
Flammability and Explosibility |
Nonflammable |
IUPAC Name: 5-(1,3-dioxo-2-benzofuran-5-yl)-2-benzofuran-1,3-dione
Isomeric SMILES: C1=CC2=C(C=C1C3=CC4=C(C=C3)C(=O)OC4=O)C(=O)OC2=O
InChIKey: WKDNYTOXBCRNPV-UHFFFAOYSA-N
InChI: InChI=1S/C16H6O6/c17-13-9-3-1-7(5-11(9)15(19)21-13)8-2-4-10-12(6-8)16(20)22-14(10)18/h1-6H
The invention discloses a method for pre...
The invention discloses a method for pre...
The article describes the synthesis and characterization of a novel polyimide derived from 3,3',4,4'-biphenyltetracarboxylic dianhydride (BPDA) and 4,4'-diaminodiphenyl ether (ODA) for high-performance applications. The authors synthesized the polyimide by a two-step process involving the preparation of BPDA dianhydride and the subsequent reaction with ODA in a solvent under reflux.
The invention discloses a method for pre...
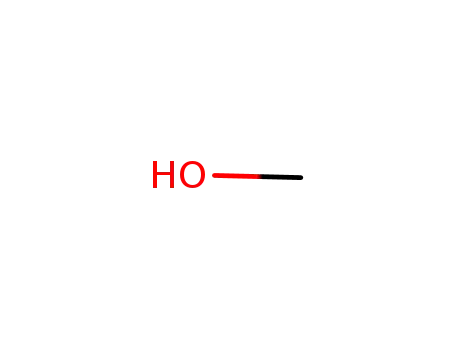
methanol

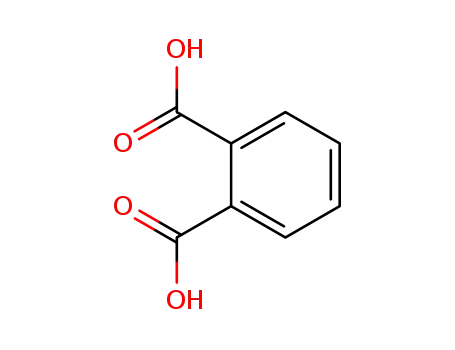
benzene-1,2-dicarboxylic acid

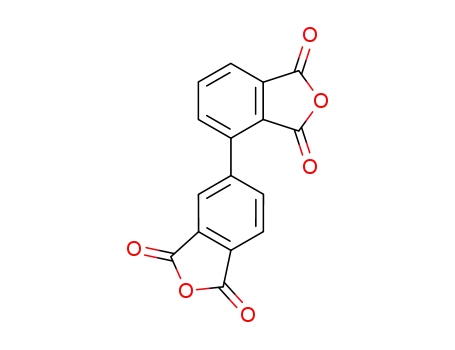
2,3',3,4'-biphenyltetracarboxylic acid dianhydride

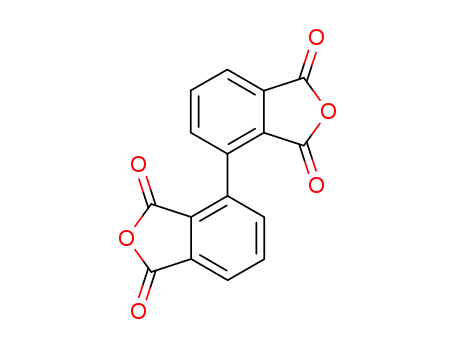
2,3,2',3'-biphenyltetracarboxylic dianhydride

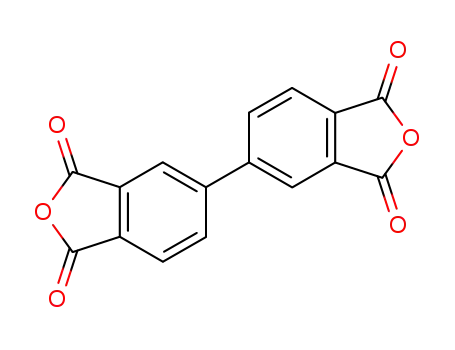
3,3',4,4'-biphenyltetracarboxylic anhydride
| Conditions | Yield |
|---|---|
|
benzene-1,2-dicarboxylic acid; With chlorine; In water; for 3h;
methanol; for 1.5h; Further stages;
|


2,3',3,4'-biphenyltetracarboxylic acid dianhydride


3,3',4,4'-biphenyltetracarboxylic anhydride
| Conditions | Yield |
|---|---|
|
|
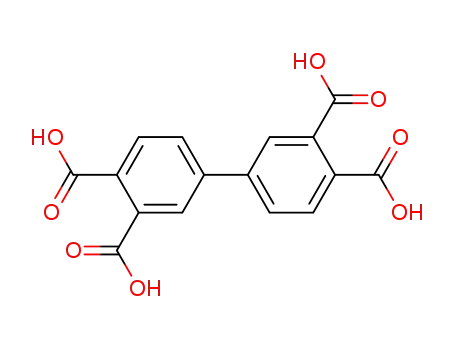
4,4'-biphthalic acid
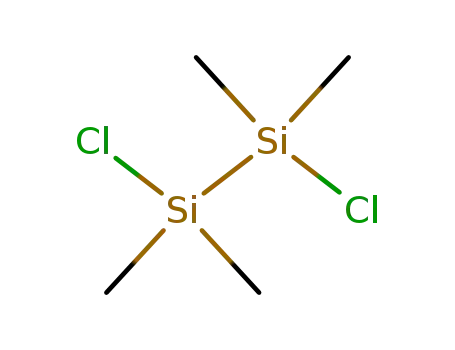
1,2-dichlorotetramethylsilane
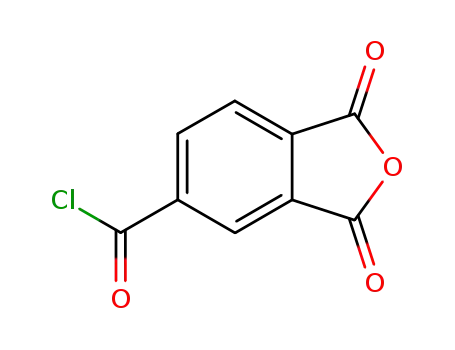
trimellitic anhydride acid chloride

3,4,3',4'-Tetramethylbiphenyl
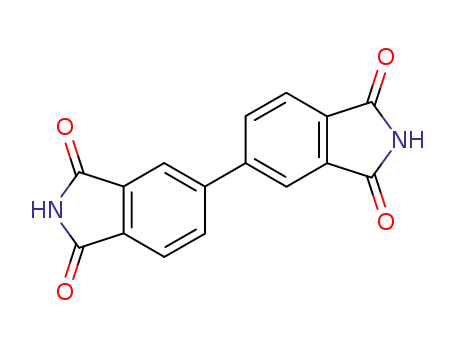
3,3’,4,4’-biphenyltetracarboxylic diimide
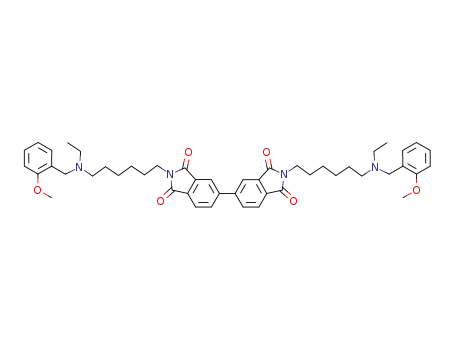
2,2'-bis-{6-[ethyl-(2-methoxybenzyl)amino]hexyl}-[5,5']biisoindolyl-1,3,1',3'-tetraone
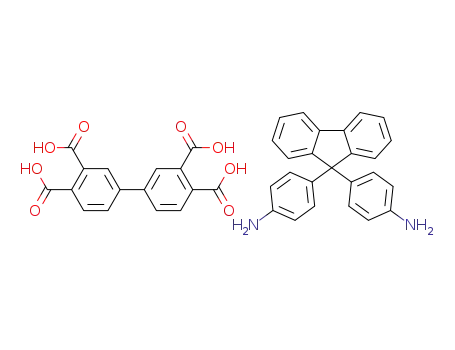
C25H20N2*C16H10O8
C32H20N2O8
CAS:138071-82-6
CAS:57280-22-5
CAS:71675-85-9
CAS:36978-41-3