- Language:English
- English

CasNo: 131875-08-6
Molecular Formula: C29H48O4
|
General Description |
Lexacalcitol is a synthetic analog of vitamin D that is primarily used for the treatment of hyperparathyroidism and secondary hyperparathyroidism in patients with chronic kidney disease. It works by binding to the vitamin D receptors in the parathyroid glands, leading to a decrease in parathyroid hormone levels and a reduction in the release of calcium and phosphorus from the bones. This helps to maintain normal levels of these minerals in the blood and prevent the development of bone disorders such as osteoporosis. Lexacalcitol is administered orally and has been shown to be effective in lowering parathyroid hormone levels and improving bone health in patients with chronic kidney disease. However, it may also cause side effects such as gastrointestinal disturbances, headaches, and skin rashes, so it should be used with caution and under the guidance of a healthcare professional. |
InChI:InChI=1/C29H48O4/c1-6-29(32,7-2)16-9-17-33-21(4)25-13-14-26-22(10-8-15-28(25,26)5)11-12-23-18-24(30)19-27(31)20(23)3/h11-12,21,24-27,30-32H,3,6-10,13-19H2,1-2,4-5H3/b22-11+,23-12-/t21-,24-,25-,26+,27+,28-/m1/s1
Eight 2-methyl substituted analogues of ...
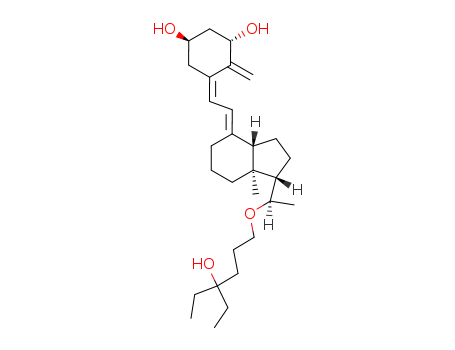
![6-((R)-1-{(1S,3aS,7aS)-4-[2-[(3S,5R)-3,5-Bis-(tert-butyl-dimethyl-silanyloxy)-2-methylene-cyclohex-(Z)-ylidene]-eth-(E)-ylidene]-7a-methyl-octahydro-inden-1-yl}-ethoxy)-3-ethyl-hexan-3-ol](/upload/2025/12/4353ac96-9f8e-4b56-b814-4504d55e8f15.png)
6-((R)-1-{(1S,3aS,7aS)-4-[2-[(3S,5R)-3,5-Bis-(tert-butyl-dimethyl-silanyloxy)-2-methylene-cyclohex-(Z)-ylidene]-eth-(E)-ylidene]-7a-methyl-octahydro-inden-1-yl}-ethoxy)-3-ethyl-hexan-3-ol

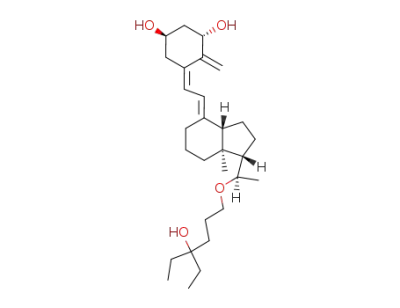
lexacalcitol
| Conditions | Yield |
|---|---|
|
With
camphor-10-sulfonic acid;
In
methanol;
at 20 ℃;
|
30% |
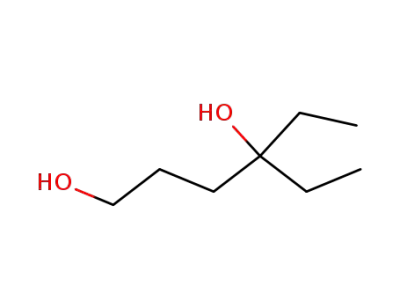
4-ethyl-hexane-1,4-diol


lexacalcitol
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 9 steps
1: 96 percent / pyridine / 1 h / 0 °C
2: 2,6-lutidine / 1 h / 0 °C
3: 92 percent / NaI / dimethylformamide / 6 h / 20 °C
4: 86 percent / KH; 18-crown-6 / tetrahydrofuran / 20 °C
5: 81 percent / TFA / CH2Cl2 / 0.75 h / 0 °C
6: 80 percent / 4 Angstroem molecular sieves; PDC / CH2Cl2 / 1 h / 20 °C
7: 33 percent / NaHMDS / tetrahydrofuran / -60 - 20 °C
8: (dba)3Pd2*CHCl3; Ph3P; Et3N / toluene / 6 h / Heating
9: 30 percent / CSA / methanol / 20 °C
With
2,6-dimethylpyridine; tris(dibenzylideneacetone)dipalladium(0) chloroform complex; dipyridinium dichromate; 18-crown-6 ether; 4 A molecular sieve; camphor-10-sulfonic acid; sodium hexamethyldisilazane; potassium hydride; triethylamine; triphenylphosphine; trifluoroacetic acid; sodium iodide;
In
tetrahydrofuran; pyridine; methanol; dichloromethane; N,N-dimethyl-formamide; toluene;
1: esterification / 2: silylation / 3: substitution / 4: etherification / 5: hydrolysis / 6: oxidation / 7: Wittig reaction / 8: cyclocondensation / 9: hydrolysis;
|

4-ethyl-hexane-1,4-diol
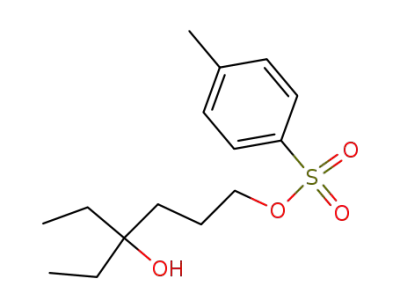
Toluene-4-sulfonic acid 4-ethyl-4-hydroxy-hexyl ester
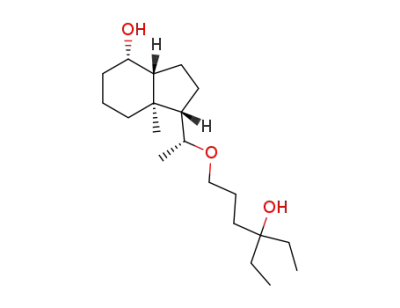
(20R)-De-A,B-24,26,27-trihomo-22-oxacholestane-8β,25-diol
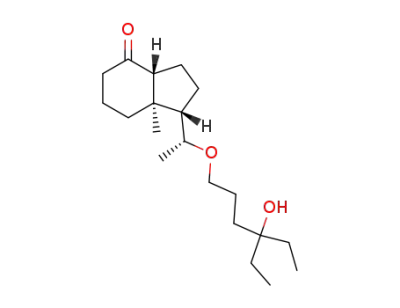
(20R)-De-A,B-24,26,27-trihomo-25-hydroxy-22-oxacholestane-8-one
CAS:138071-82-6
CAS:57280-22-5
CAS:1692-25-7
CAS:34408-14-5