- Language:English
- English
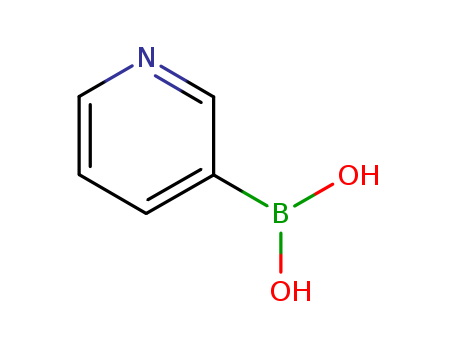

CasNo: 1692-25-7
Molecular Formula: C5H6BNO2
Appearance: Light yellow powder
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 64, p. 3846, 1999 DOI: 10.1021/jo9819279Tetrahedron Letters, 43, p. 4285, 2002 |
InChI:InChI=1/C5H6BNO2/c8-6(9)5-2-1-3-7-4-5/h1-4,8-9H
Water soluble zwitterionic fluorescent c...
A new M2L4 molecular capsule with an aro...
Disclosed herein is a novel simple, shor...
The invention provides a 2-amino-5-heter...
PURPOSE: A compound is provided to impro...
The invention provides a preparation met...
3-Bromopyridine

Triisopropyl borate

3-pyridylboronic acid
| Conditions | Yield |
|---|---|
|
3-Bromopyridine;
With
n-butyllithium;
In
tetrahydrofuran; hexanes; toluene;
at -60 - -50 ℃;
for 0.75h;
Triisopropyl borate;
With
hydrogenchloride;
In
tetrahydrofuran; hexanes; water; toluene;
at -50 - -15 ℃;
|
73% |
|
With
n-butyllithium;
In
tetrahydrofuran; hexane; toluene;
at -40 ℃;
for 1.5h;
|
|
|
3-Bromopyridine;
With
n-butyllithium;
In
tetrahydrofuran; hexane; toluene;
at -60 - -50 ℃;
for 0.583333h;
Triisopropyl borate;
In
tetrahydrofuran; hexane; toluene;
at -15 ℃;
|

3-Bromopyridine


3-pyridylboronic acid
| Conditions | Yield |
|---|---|
|
3-Bromopyridine;
With
isopropylmagnesium chloride;
In
tetrahydrofuran;
at 20 ℃;
for 2h;
With
tris(trimethylsilyl)borate;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 24h;
With
hydrogenchloride;
at 0 ℃;
pH=6 - 7;
|
75% |
|
3-Bromopyridine;
With
n-butyllithium;
In
tetrahydrofuran; hexane; toluene;
at -50 ℃;
With
Triisopropyl borate;
In
tetrahydrofuran; hexane; toluene;
at -50 - -15 ℃;
With
sodium hydroxide;
In
tetrahydrofuran; hexane; toluene;
|
73% |
|
With
n-butyllithium; Triisopropyl borate;
In
tetrahydrofuran;
at -78 ℃;
for 3h;
|
71% |
|
3-Bromopyridine;
With
n-butyllithium;
In
hexanes; toluene;
at -60 - -50 ℃;
With
Triisopropyl borate;
In
tetrahydrofuran; hexanes; toluene;
at -50 - -15 ℃;
With
hydrogenchloride;
In
tetrahydrofuran; hexanes; water; toluene;
|
50% |
|
3-Bromopyridine;
With
n-butyllithium;
In
diethyl ether; hexane;
With
Trimethyl borate;
In
diethyl ether; hexane;
|
|
|
With
n-butyllithium; Triisopropyl borate;
In
tetrahydrofuran; hexane; toluene;
at -40 - -20 ℃;
|
|
|
3-Bromopyridine;
With
n-butyllithium; Triisopropyl borate;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 4h;
In
tetrahydrofuran; hexane;
pH=7;
Alkaline conditions;
|
|
|
3-Bromopyridine;
With
n-butyllithium;
In
diethyl ether; hexane;
at -80 ℃;
for 0.5h;
Inert atmosphere;
With
Trimethyl borate;
In
diethyl ether; hexane;
at -80 - 20 ℃;
for 24h;
Inert atmosphere;
|
|
|
With
n-butyllithium; Triisopropyl borate;
In
tetrahydrofuran;
|
|
|
Multi-step reaction with 2 steps
1.1: n-butyllithium / toluene; hexane / -60 - -50 °C
1.2: 0.03 h
2.1: hydrogenchloride / water / -15 °C
With
hydrogenchloride; n-butyllithium;
In
hexane; water; toluene;
|
|
|
Multi-step reaction with 2 steps
1: n-butyllithium / tetrahydrofuran / 0.33 h / -30 °C
2: water; sodium hydroxide / 3 h / 30 °C
With
n-butyllithium; water; sodium hydroxide;
In
tetrahydrofuran;
|
|
|
With
n-butyllithium; Triisopropyl borate;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 4h;
pH=~ 7;
|

3-Bromopyridine
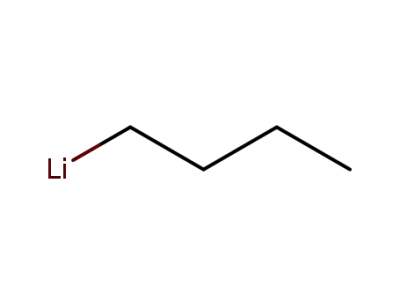
n-butyllithium
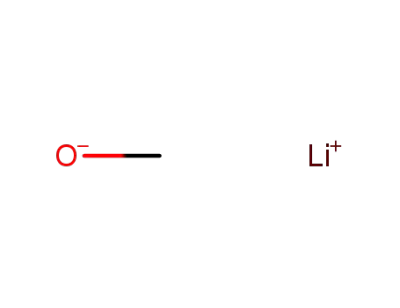
lithium methanolate
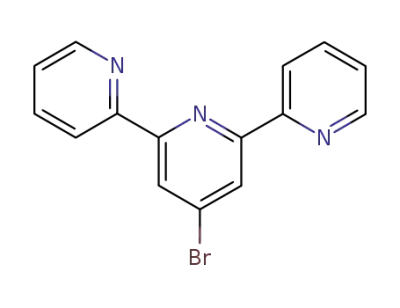
4'-bromo-2,2':6',2''-terpyridine
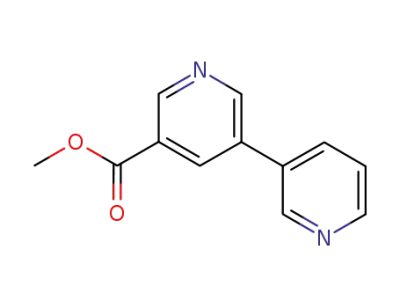
methyl <3,3'-bipyridine>-5-carboxylate
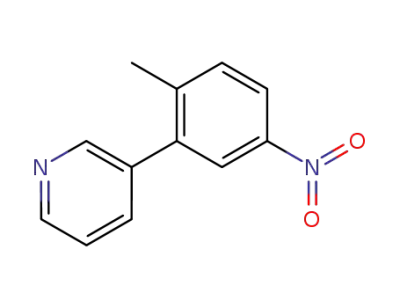
3-(2-methyl-5-nitro-phenyl)-pyridine
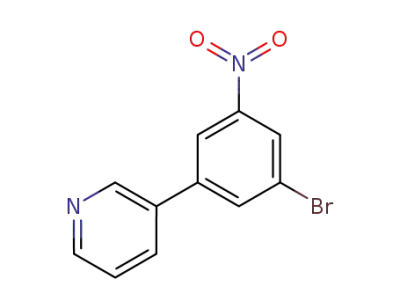
3-(3-bromo-5-nitro-phenyl)-pyridine
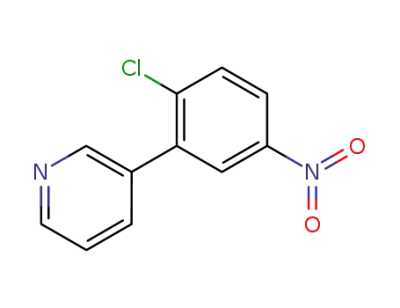
3-(2-chloro-5-nitro-phenyl)-pyridine
CAS:1953-04-4
CAS:20826-04-4
CAS:134404-52-7
CAS:131875-08-6