- Language:English
- English
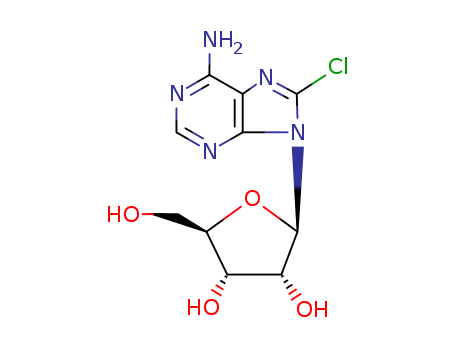

CasNo: 34408-14-5
Molecular Formula: C10H12ClN5O4
|
General Description |
8-Chloroadenosine is a synthetic derivative of adenosine that has been studied for its potential as an anticancer agent. It is a nucleoside analog that has been shown to inhibit the growth of cancer cells by interfering with their DNA and RNA synthesis. 8-Chloroadenosine has also been found to induce apoptosis, or programmed cell death, in cancer cells, making it a promising candidate for chemotherapy treatment. Additionally, it has been investigated for its potential to enhance the effectiveness of other chemotherapy drugs when used in combination. Overall, 8-Chloroadenosine shows promise as a novel therapeutic agent for the treatment of various types of cancer. |
InChI:InChI=1/C10H12ClN5O4/c11-10-15-4-7(12)13-2-14-8(4)16(10)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,13,14)
-
The present invention relates to chemica...
ecto-5′-Nucleotidase (eN, CD73) catalyze...
6,8-Disubstituted purines which can be u...
8-Chloroadenosine (8-Cl-Ado) has shown p...
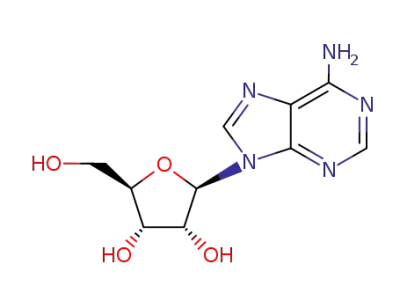
adenosine

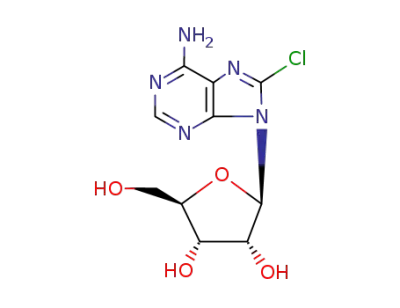
8-chloroadenosine
| Conditions | Yield |
|---|---|
|
With
N-chloro-succinimide; acetic acid;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 48h;
Concentration;
Reagent/catalyst;
|
52% |
|
With
hydrogenchloride; 3-chloro-benzenecarboperoxoic acid;
In
N,N-dimethyl acetamide;
for 3.5h;
Ambient temperature;
|
50% |
|
With
hydrogenchloride; 3-chloro-benzenecarboperoxoic acid;
In
N,N-dimethyl acetamide;
at 0 - 25 ℃;
for 2.5h;
|
39% |
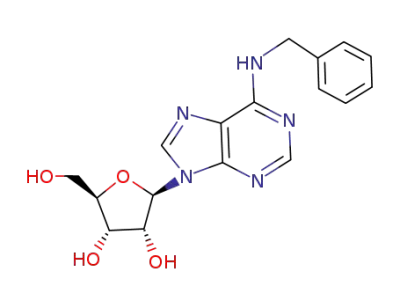
N6-Benzyladenosine


8-chloroadenosine

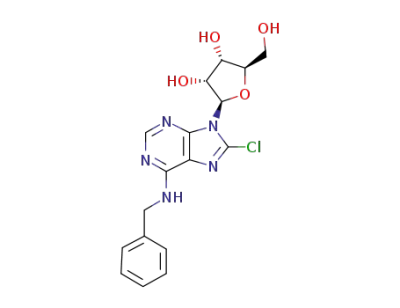
6-benzylamino-8-chloro-9-(β-D-ribofuranosyl)purine
| Conditions | Yield |
|---|---|
|
With
N-chloro-succinimide;
In
N,N-dimethyl-formamide;
at 45 ℃;
for 18h;
|
20% |

adenosine
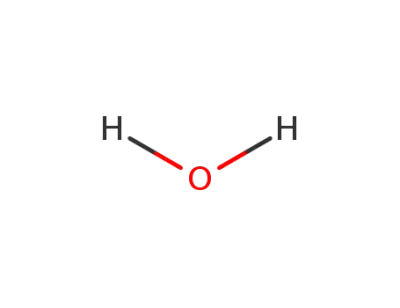
water

N6-Benzyladenosine
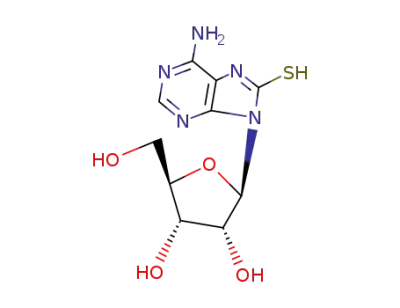
8-mercaptoadenosine
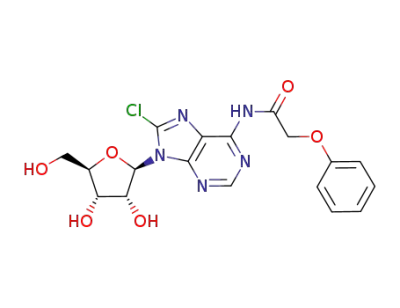
N6-phenoxyacetyl-8-chloroadenosine
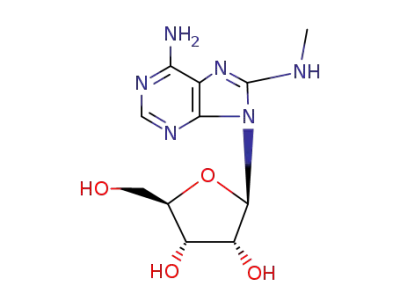
8-Methylaminoadenosine
CAS:38083-17-9
CAS:120011-70-3
CAS:131875-08-6
CAS:302904-82-1