- Language:English
- English
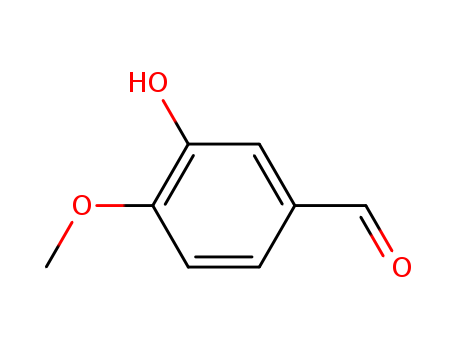

CasNo: 621-59-0
Molecular Formula: C8H8O3
Appearance: light tan cyrstalline solid
|
Preparation |
Synthesis of isovanillin: 4-Hydroxybenzaldehyde is used as raw material, firstly react with bromine to obtain 3-bromo-4-hydroxybenzaldehyde, then react with methyl iodide to obtain 3-bromo-4-methoxybenzaldehyde, and then hydrolyzed under the action of sodium hydroxide and cuprous chloride to produce isovanillin. The yield was 64.1%. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 45, p. 1596, 1980 DOI: 10.1021/jo01297a010 |
|
Flammability and Explosibility |
Notclassified |
|
Purification Methods |
Crystallise isovaniline from H2O or *C6H6. The oxime has m 147o. [Beilstein 8 IV 1764.] |
|
General Description |
Isovanillin, also known as 3-hydroxy-4-methoxybenzaldehyde, is a versatile aromatic aldehyde used in organic synthesis, particularly in the preparation of sweetening agents and bioactive compounds. It serves as a key intermediate in the copper(I)-catalyzed synthesis of 1,4-benzodioxines, yielding sweetening derivatives significantly sweeter than sucrose. Additionally, it has been employed in the synthesis of bactericidal arylidenedeoxyvasicinones, though these compounds exhibited only moderate to low antimicrobial activity. Its functional groups allow for diverse chemical modifications, making it valuable in pharmaceutical and flavoring applications. |
|
Application |
Isovanillin is a kind of fragrance and isomer of vanillin. it has unique properties than vanillin, its fragrance can change with the change of ambient temperature, so it is especially suitable for special cosmetics and some special fragrance industries. Isovanillin is a selective inhibitor of aldehyde oxidase. It is not a substrate of that enzyme, and is metabolized by aldehyde dehydrogenase into isovanillic acid, which could make it a candidate drug for use in alcohol aversion therapy. |
InChI:InChI=1/C8H8O3/c1-11-8-3-2-6(5-9)4-7(8)10/h2-5,10H,1H3
Electron-rich phenolic substrates can be...
The invention provides a method for synt...
Demethylating methyl phenyl ethers is ch...
The invention provides a preparation met...
piperonal

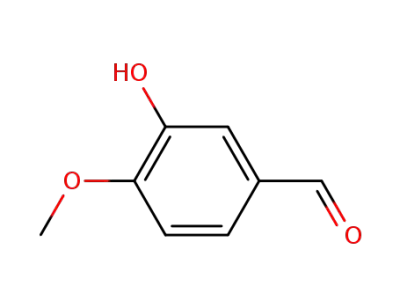
isovanillin

piperonol

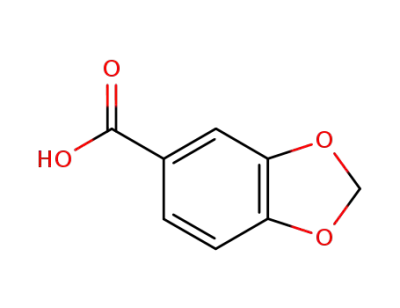
Piperonylic acid

![4-(Benzo[1,3]dioxol-5-ylmethoxy)-3-hydroxy-benzaldehyde](/upload/2025/12/8ab1a5e3-1b80-4c6c-bbdd-f18ed4c4b895.png)
4-(Benzo[1,3]dioxol-5-ylmethoxy)-3-hydroxy-benzaldehyde
| Conditions | Yield |
|---|---|
|
With
sodium methylate;
In
dimethyl sulfoxide;
at 150 ℃;
|
18.8% 17.6% 14.5% 18% |
|
With
sodium methylate;
In
dimethyl sulfoxide;
at 150 ℃;
|
18.8% 17.6% 18% 14.5% |

piperonal

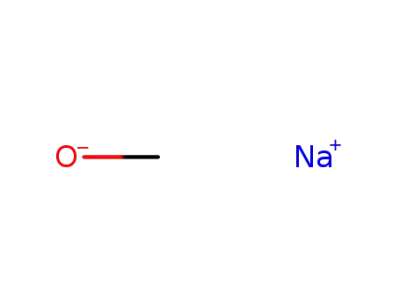
sodium methylate


isovanillin


piperonol


Piperonylic acid

![4-(Benzo[1,3]dioxol-5-ylmethoxy)-3-hydroxy-benzaldehyde](/upload/2025/12/8ab1a5e3-1b80-4c6c-bbdd-f18ed4c4b895.png)
4-(Benzo[1,3]dioxol-5-ylmethoxy)-3-hydroxy-benzaldehyde
| Conditions | Yield |
|---|---|
|
In
dimethyl sulfoxide;
at 150 ℃;
|
17.6% 18% 14.5% 18.8% |
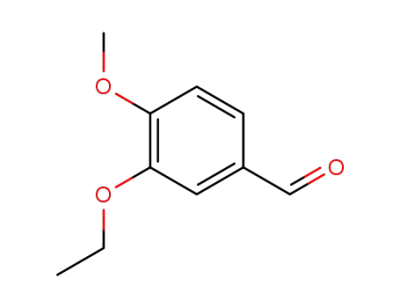
3-ethoxy-4-methoxybenzaldehyde
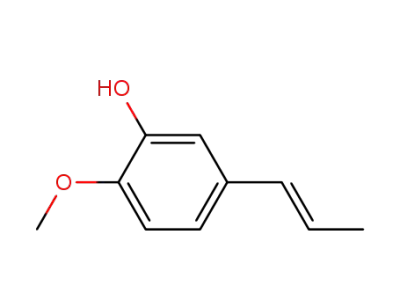
Isochavibetol
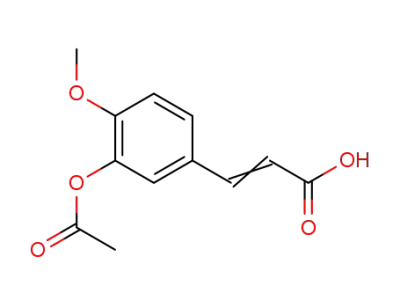
3-(3-acetoxy-4-methoxyphenyl)acrylic acid
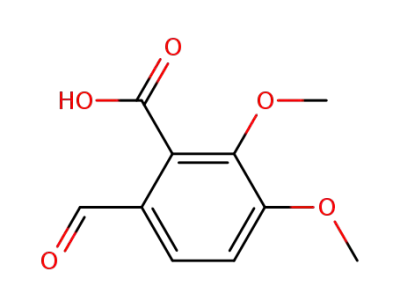
opianic acid
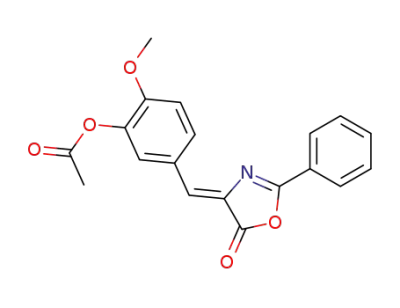
(Z)-2-methoxy-5-((5-oxo-2-phenyloxazol-4(5H)-ylidene)methyl)phenyl acetate
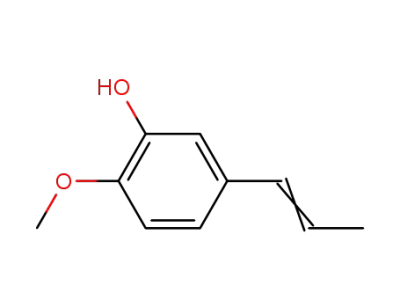
Isochavibetol

3-ethoxy-4-methoxybenzaldehyde
(E)-1-(2,4-dihydroxyphenyl)-3-(3-hydroxy-4-methoxyphenyl)-prop-2-en-1-one
CAS:138071-82-6
CAS:57280-22-5
CAS:21343-40-8
CAS:5116-24-5