- Language:English
- English
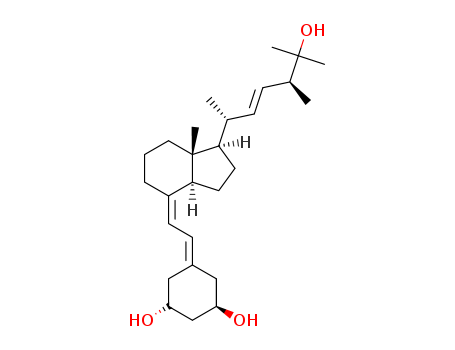

CasNo: 131918-61-1
Molecular Formula: C27H44O3
|
Drug interactions |
Potentially hazardous interactions with other drugs None known |
|
Metabolism |
Extensively metabolised via hepatic and non-hepatic pathways to form two relatively inactive metabolites. After oral administration of 3 H-paricalcitol, only about 2% of the dose was eliminated unchanged in the faeces, and no parent drug found in the urine. Approximately 70% of the radioactivity was eliminated in the faeces and 18% was recovered in the urine. Most of the systemic exposure was from the parent drug. |
|
Brand name |
Zemplar (Abbott). |
InChI:InChI=1/C27H44O3/c1-18(8-9-19(2)26(3,4)30)24-12-13-25-21(7-6-14-27(24,25)5)11-10-20-15-22(28)17-23(29)16-20/h8-11,18-19,22-25,28-30H,6-7,12-17H2,1-5H3/b9-8+,21-11+/t18-,19+,22-,23-,24-,25+,27-/m1/s1
A novel strategy was developed for the p...
The invention provides a preparation met...
The invention discloses a preparation me...
The invention provides a purification me...
The invention discloses a preparation me...
C29H46O4

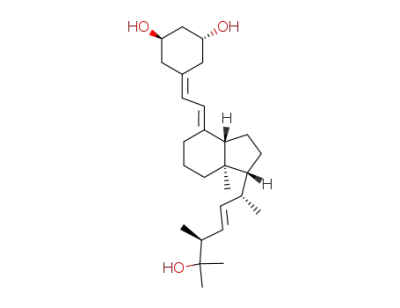
paricalcitol
| Conditions | Yield |
|---|---|
|
With
water; sodium hydroxide;
In
ethanol;
at 20 - 25 ℃;
for 1.33333h;
|
89.5% |
C39H72O3Si2


paricalcitol
| Conditions | Yield |
|---|---|
|
With
10-camphorsulfonic acid;
In
methanol; chloroform;
at 20 ℃;
for 1.5h;
|
57% |
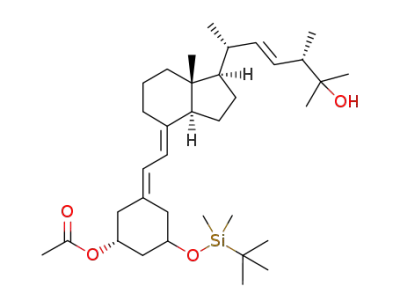
C35H60O4Si
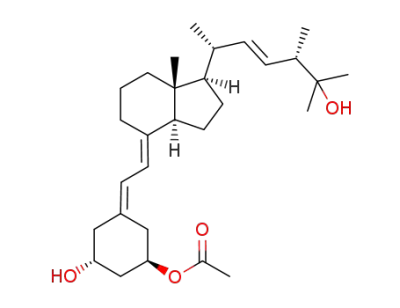
C29H46O4
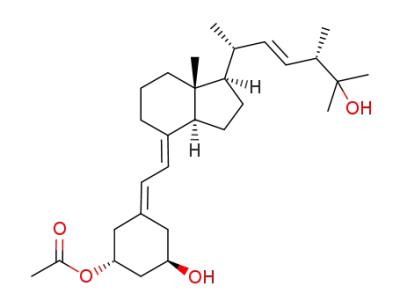
C29H46O4
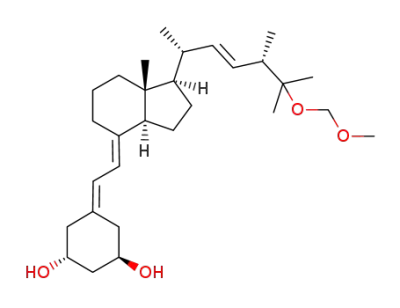
1α-hydroxy-25-methoxymethyloxy-19-norvitamin D2
CAS:138071-82-6
CAS:57280-22-5
CAS:69123-90-6
CAS:3900-89-8