- Language:English
- English
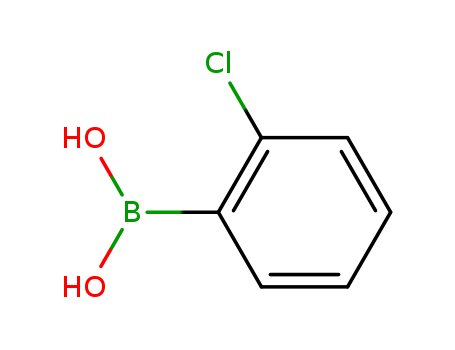

CasNo: 3900-89-8
Molecular Formula: C6H6BClO2
Appearance: white crystalline powder
InChI:InChI=1/C6H6BClO2/c8-6-4-2-1-3-5(6)7(9)10/h1-4,9-10H
The invention relates to the technical f...
Owing to the unusual reactivity of dialk...
A mild aqueous protocol for palladium ca...
The generally accepted monoacyloxyboron ...
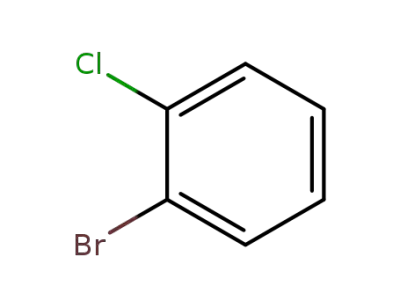
2-bromo-1-chlorobenzene

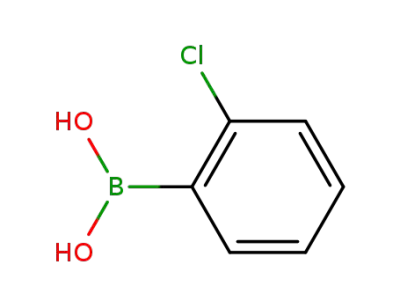
2-Chlorobenzeneboronic acid
| Conditions | Yield |
|---|---|
|
With
n-butyllithium; Triisopropyl borate;
In
tetrahydrofuran; hexane; toluene;
at -70 ℃;
for 1.5h;
Inert atmosphere;
|
98% |
|
2-bromo-1-chlorobenzene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
Inert atmosphere;
With
Trimethyl borate;
In
tetrahydrofuran; hexane;
Inert atmosphere;
|
56% |
|
With
n-butyllithium; Triisopropyl borate;
In
tetrahydrofuran; hexane; toluene;
at -70 - -20 ℃;
|
100 % Chromat. |

methanol


diisopropylamine borane


2-bromo-1-chlorobenzene


2-Chlorobenzeneboronic acid
| Conditions | Yield |
|---|---|
|
diisopropylamine borane;
With
magnesium; phenylmagnesium bromide;
In
tetrahydrofuran;
at 20 ℃;
for 0.166667h;
2-bromo-1-chlorobenzene;
In
tetrahydrofuran;
at 70 ℃;
methanol;
Further stages;
|
85% |
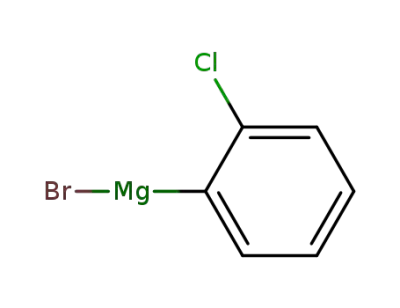
2-chlorophenylmagnesium bromide

triisobutyl borate

boric acid tributyl ester

2-bromo-1-chlorobenzene
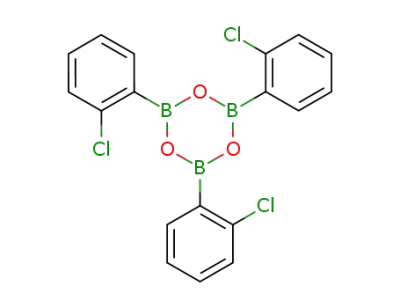
tris(2-chloro phenyl)boroxine
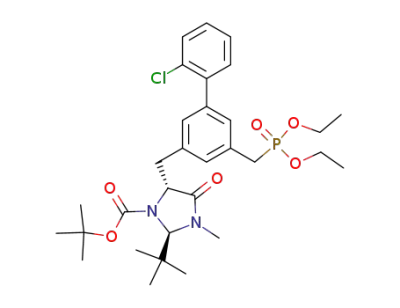
1,1-dimethylethyl (2R,5R)-5-<<2'-chloro-5-<(diethoxyphopsphoryl)methyl>(1,1'-biphenyl)-3-yl>methyl>-2-(1,1-dimethylethyl)-3-methyl-4-oxoimidazolidine-1-carboxylate
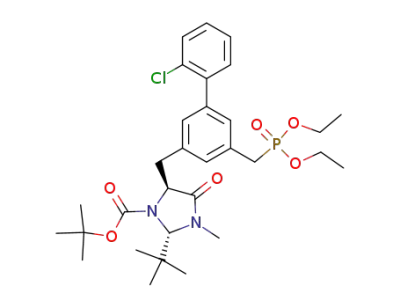
1,1-dimethylethyl (2S,5S)-5-<<2'-chloro-5-<(diethoxyphopsphoryl)methyl>(1,1'-biphenyl)-3-yl>methyl>-2-(1,1-dimethylethyl)-3-methyl-4-oxoimidazolidine-1-carboxylate
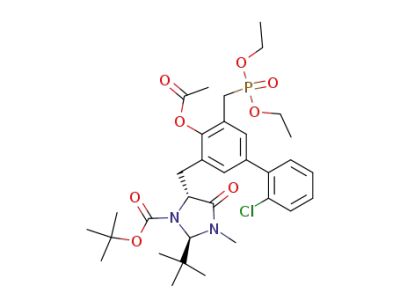
(2R,5R)-5-[4-Acetoxy-2'-chloro-5-(diethoxy-phosphorylmethyl)-biphenyl-3-ylmethyl]-2-tert-butyl-3-methyl-4-oxo-imidazolidine-1-carboxylic acid tert-butyl ester
CAS:2173554-83-9
CAS:131918-61-1
CAS:32222-06-3