- Language:English
- English
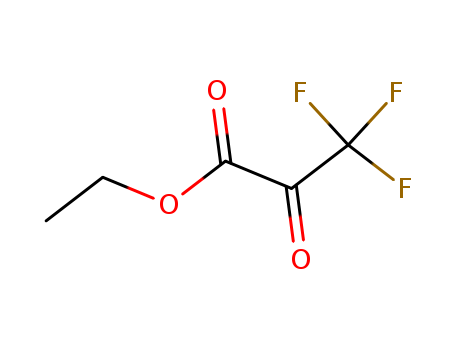

CasNo: 13081-18-0
Molecular Formula: C5H5F3O3
Appearance: Yellow to pale yellow clear liquid
|
Chemical Properties |
Clear pale yellow liquid |
|
Uses |
Ethyl Trifluoropyruvate is used as a reagent in the preparation of potential biologically active compounds such as in the highly enantioselective organocatalytic hydroxyalkylation of indoles . |
|
General Description |
Ethyl 3,3,3-trifluoropyruvate is a trifluoromethylated compound. Enantioselective Friedel–Crafts alkylation of simple phenols and indoles with ethyl 3,3,3-trifluoropyruvate under different reaction conditions have been reported. |
InChI:InChI=1/C4H3F3O3/c1-10-3(9)2(8)4(5,6)7/h1H3
Reaction of zinc with bromotrifluorometh...
It is possible to produce a fluorine-con...
The thermal stability and dimerization r...
A Barbier procedure, under moderate pres...
A process for the preparation of a perfl...
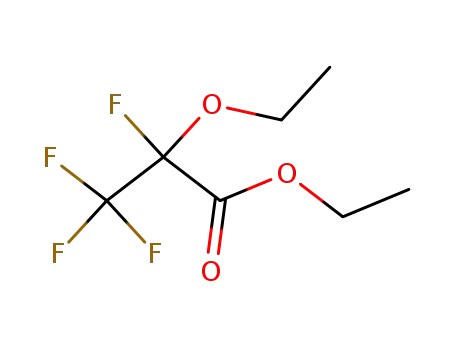
2-fluoro-2-ethoxy-trifluoropropionic acid ethyl ester

1-fluoroethane

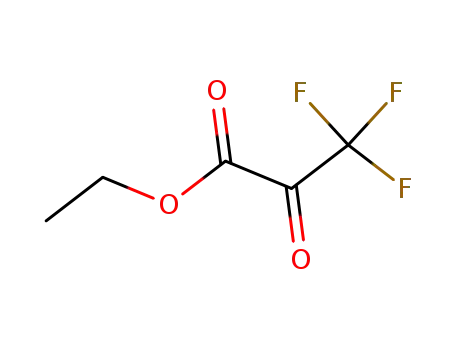
ethyl-3,3,3-trifluoropyruvate
| Conditions | Yield |
|---|---|
|
antimony pentafluoride;
In
neat (no solvent);
|
83% |
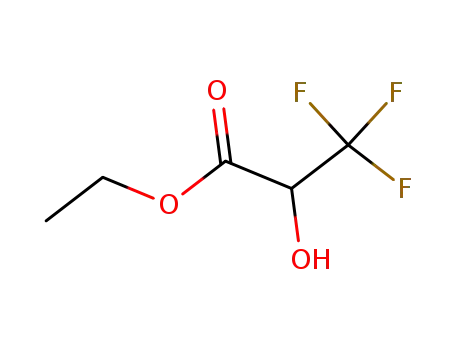
ethyl 3,3,3-trifluoro-2-hydroxypropanoate


ethyl-3,3,3-trifluoropyruvate
| Conditions | Yield |
|---|---|
|
With
sodium hypochlorite pentahydrate;
In
acetonitrile;
at 20 ℃;
for 0.5h;
Reagent/catalyst;
Solvent;
|
69% |
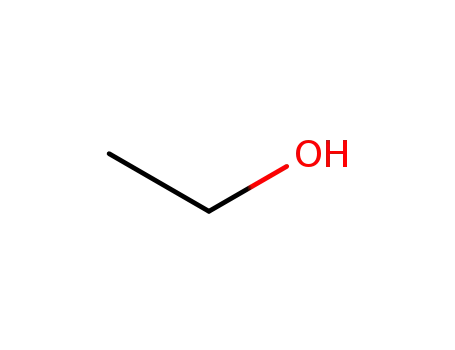
ethanol
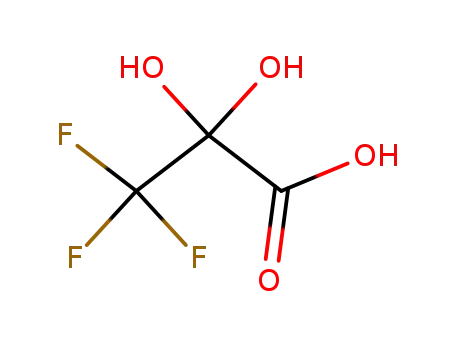
α,α-dihydroxytrifluoropropionic acid

2-fluoro-2-ethoxy-trifluoropropionic acid ethyl ester
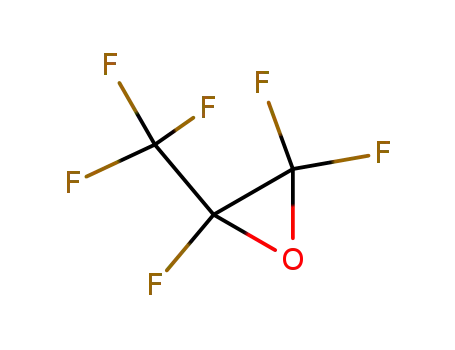
Hexafluoropropene oxide
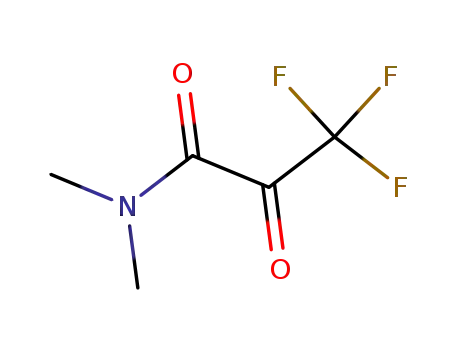
3,3,3-Trifluoro-N,N-dimethyl-2-oxo-propionamide
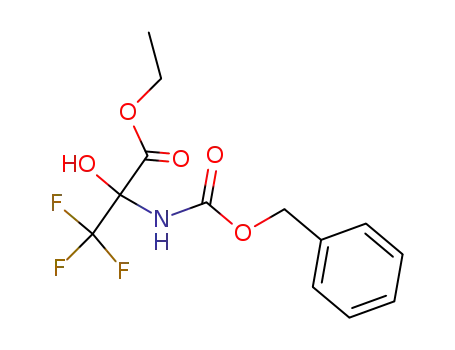
ethyl 2-(((benzyloxy)carbonyl)amino)-3,3,3-trifluoro-2-hydroxypropanoate
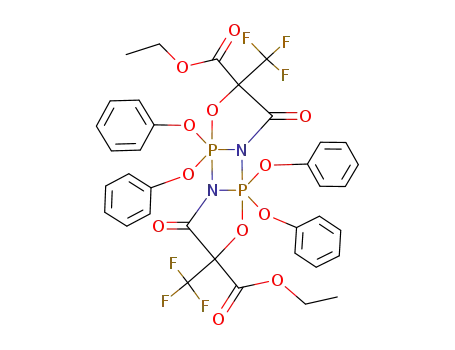
diethyl 5,10-dioxo-2,2,7,7-tetraphenoxy-4,9-bis(trifluoromethyl)-3,8-dioxa-1,6-diaza-2λ5,7λ5-diphosphatricyclo<5.3.0.02,6>decane-4,9-dicarboxylate
3,6-Bis(ethoxycarbonyl)-1,1-dimethoxy-4-phenyl-3,6-bis(trifluoromethyl)-2,7-dioxa-1-phosphabicyclo<3.2.0>hept-4-ene
CAS:99627-05-1
CAS:863329-66-2
CAS:1187-93-5
CAS:13089-11-7