- Language:English
- English
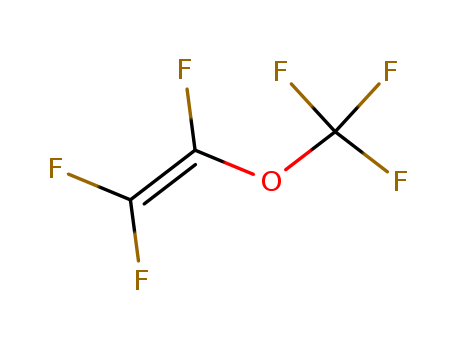

CasNo: 1187-93-5
Molecular Formula: C3F6O
Appearance: Colorless gas
|
General Description |
A colorless gas. Heavier than air. Easily liquefied. Contact with the liquid may cause frost bite by evaporative cooling. May asphyxiate by displacing air. A flame may flash back from the source of ignition to the source of a leak. Under prolonged exposure to fire or heat containers may rupture violently and rocket. 1,1,2-trifluoro-2-(trifluoromethoxy)ethene is a chemical compound that belongs to the class of fluoroorganic compounds. It is also known as trifluoromethoxydifluoroethylene or TMDFE. |
| Uses | The compound contains a trifluoromethoxy group and a double bond between two carbon atoms, with two fluorine atoms and one hydrogen atom attached to each carbon atom. TMDFE is a colorless gas with a sweet odor and is used in various industrial applications, including as a refrigerant, solvent, and in the production of fluorinated chemicals. The compound's unique chemical properties make it useful in a variety of fields, including pharmaceuticals, agrochemicals, and materials science. |
|
Air & Water Reactions |
Highly flammable. |
|
Reactivity Profile |
Trifluoromethyl trifluorovinyl ether oxidizes readily in air to form unstable peroxides that may explode spontaneously. |
|
Health Hazard |
Vapors may cause dizziness or asphyxiation without warning. Some may be irritating if inhaled at high concentrations. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating and/or toxic gases. |
|
Flammability and Explosibility |
Flammable |
IUPAC Name: 1,1,2-trifluoro-2-(trifluoromethoxy)ethene
Isomeric SMILES: C(=C(F)F)(OC(F)(F)F)F
InChIKey: BLTXWCKMNMYXEA-UHFFFAOYSA-N
InChI: InChI=1S/C3F6O/c4-1(5)2(6)10-3(7,8)9
The reaction of methyl hypofluorite (MeO...
The thermal rearrangement of trifluoromethyl trifluorovinyl ether (MVE) to pentafluoropropionyl fluoride (PPF) under pressure with and without radical initiators has been studied. The isomerization of trifluoromethyl trifluorovinyl ether to pentafluoropropionyl fluoride is a radical chain reaction where the propagating species is the CF3 radical.
The present invention relates to a proce...
Trifluoromethyl trifluorovinyl ether (I) and sulfide (II) were prepared by dehalogenation of the corresponding 1,2-dichlorotrifluoroethyl compounds. The polymerization behavior of these, along with the known trifluoroethyl trifluorovinyl ether (III) was examined.
The invention pertains to a method for t...

perfluoro(dimethyl-2-methoxybutylamine)

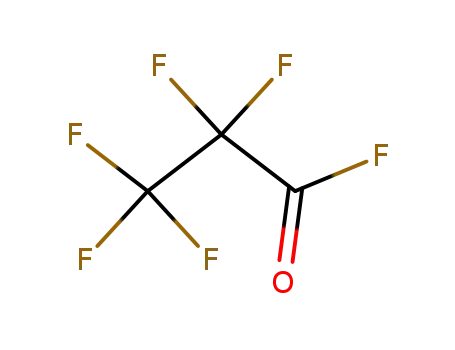
perfluoropropanoyl fluoride

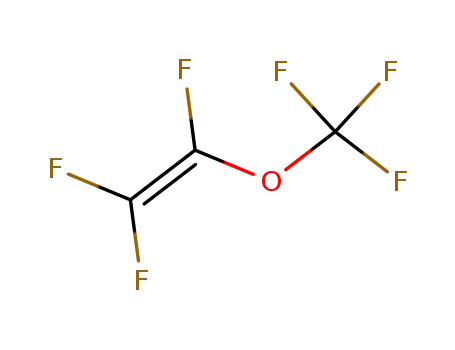
trifluoromethyl trifluorovinyl ether

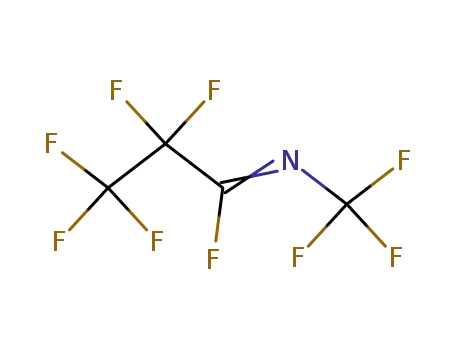
perfluoro(2-aza-2-pentene)

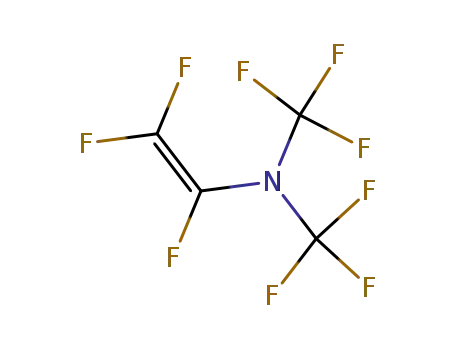
perfluoro(N,N-dimethyl trifluorovinylamine)
| Conditions | Yield |
|---|---|
|
platinum; at 590 ℃; Further byproducts given;
|
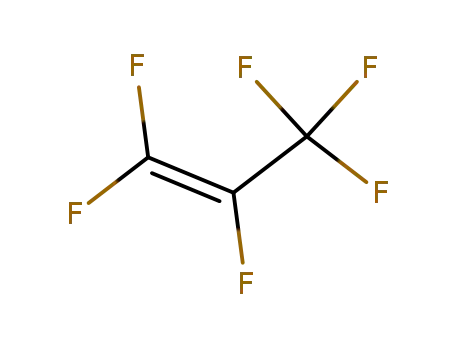
perfluoropropylene

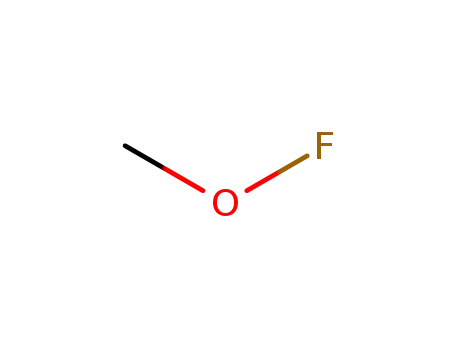
methyl hypofluorite


trifluoromethyl trifluorovinyl ether

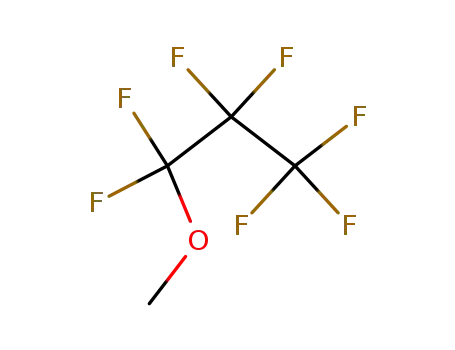
heptafluoropropyl methyl ether
| Conditions | Yield |
|---|---|
|
With sodium fluoride; In [D3]acetonitrile;
|
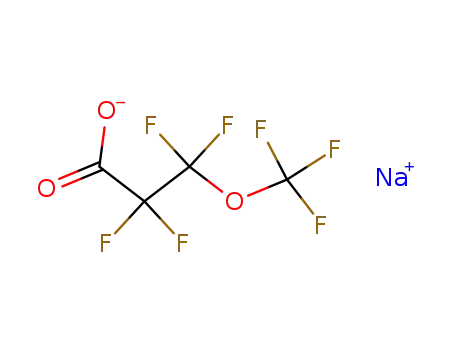
sodium perfluoro-β-methoxypropionate
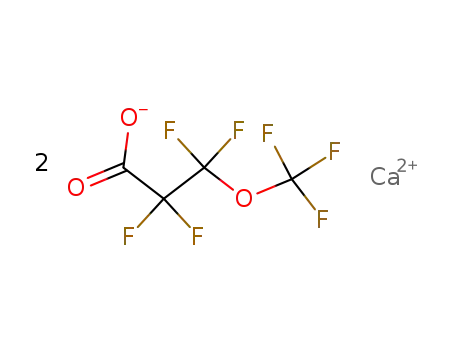
calcium perfluoro-β-methoxypropionate

perfluoro(dimethyl-2-methoxybutylamine)
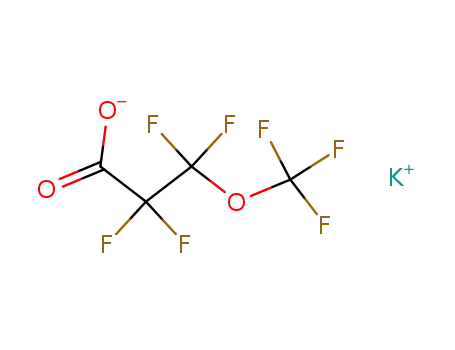
potassium perfluoro-β-methoxypropionate
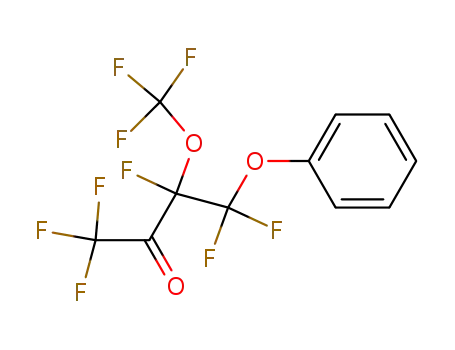
4-phenoxy-3-(trifluoromethoxy)-1,1,1,3,4,4-hexafluorobutan-2-one
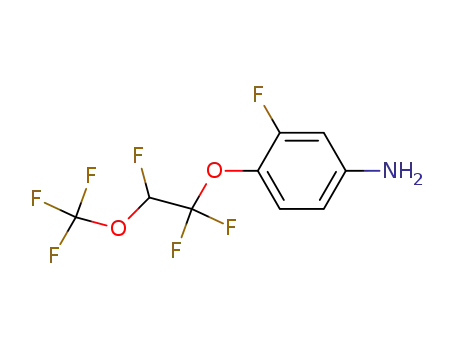
3-Fluoro-4-(1,1,2-trifluoro-2-trifluoromethoxy-ethoxy)-phenylamine
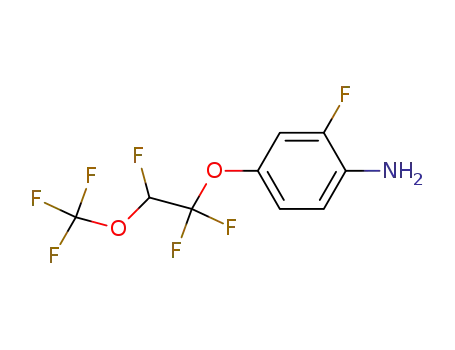
2-fluoro-4-[1,1,2-trifluoro-2-(trifluoromethoxy)ethoxy]-aniline
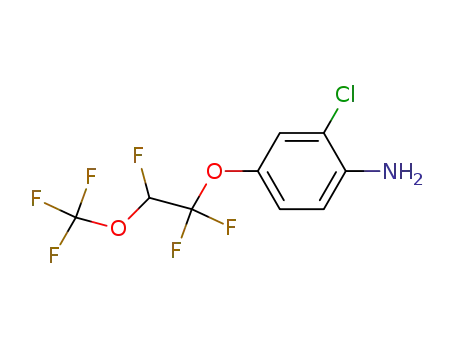
2-chloro-4-[1,1,2-trifluoro-2-(trifluoromethoxy)-ethoxy]-aniline
CAS:99627-05-1
CAS:863329-66-2
CAS:120014-07-5
CAS:13081-18-0