- Language:English
- English
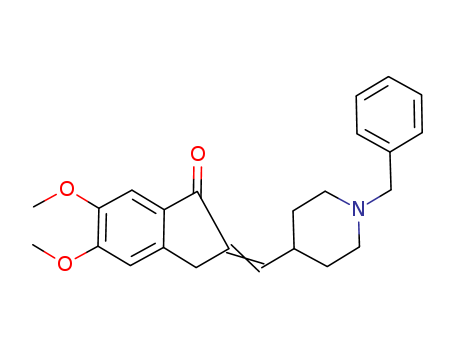

CasNo: 120014-07-5
Molecular Formula: C24H27NO3
|
Synthesis |
A solution of 5,6-dimethoxy-indanone (19 g, 0.10 mol) in methanol (8 mL) is stirred under inert atmosphere at room temperature. Slowly add NaOH flakes (12.8 g, 0.32 mol) followed by N-benzyl-piperidine-4-carboxaldehyde (20.2 g, 0.10 mol) to the reaction mixture. The mixture was stirred at room temperature for 3 h and progress of the reaction was monitored by TLC (hexane:ethyl acetate; 1:1). Once the reaction is complete, the solid formed was filtered, washed with 5 % acetic acid and then with methanol and dried. The obtained solid (34 g) was taken into a round bottom flask and refluxed with DMF (50 mL). Gradually cooled to room temperature and stirred for 2 h, filtered the solid formed, wash with chilled methanol to afford a pale yellow crystalline solid 1-Benzyl-4-(5,6-dimethoxy-1-oxoindan-2-ylindenemethyl)piperidine (32.0 g, 84 %); m.p.: 175-177 °C. |
|
Uses |
An impurity of Donepezil; an intermediate as an anti-Alzheimer agent. 1-Benzyl-4-(5,6-dimethoxy-1-oxoindan-2-ylindenemethyl)piperidine is a chemical compound with potential pharmacological properties. It belongs to the class of piperidine derivatives and contains a benzyl group, a piperidine ring, and an indane moiety with two methoxy and one oxo substituents. The compound has been studied for its potential as an antipsychotic agent due to its ability to modulate dopamine and serotonin receptors in the brain. |
IUPAC Name: (2E)-2-[(1-benzylpiperidin-4-yl)methylidene]-5,6-dimethoxy-3H-inden-1-one
Isomeric SMILES: COC1=C(C=C2C(=C1)C/C(=C\C3CCN(CC3)CC4=CC=CC=C4)/C2=O)OC
InChIKey: LPMOTUSFDTTWJL-UDWIEESQSA-N
InChI: InChI=1S/C24H27NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,12,14-15,17H,8-11,13,16H2,1-2H3/b20-12+
A simple, efficient and highly economic ...
...
The invention discloses a purification m...
This paper describes a simple, efficient...
The invention discloses a preparation me...
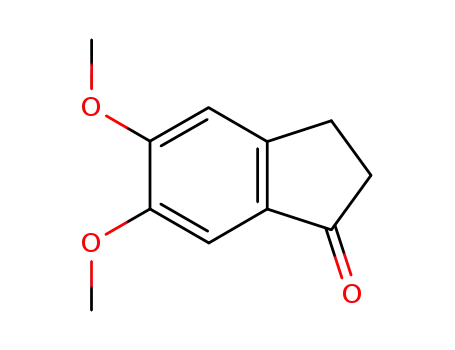
5,6-dimethoxy-1-indanone

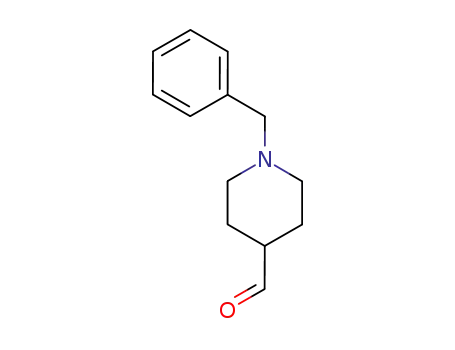
N-benzyl-4-formylpiperidine

1-benzyl-4-<(5,6-dimethoxy-1-oxoindan-2-ylidenyl)methyl>piperidine
| Conditions | Yield |
|---|---|
|
With potassium hydroxide; N-benzyl-N,N,N-triethylammonium chloride; In water; toluene; at 20 ℃; Product distribution / selectivity; Inert atmosphere; Reflux;
|
100% |
|
With sodium methylate; In methanol; ethanol; at 79 ℃; for 1.58333h; Product distribution / selectivity; Heating / reflux;
|
93.4% |
|
With sodium methylate; In methanol; at 66 ℃; for 1h; Product distribution / selectivity; Heating / reflux;
|
93.9% |
|
With sodium ethanolate; In ethanol; at 75 - 79 ℃; for 1.2h; Product distribution / selectivity; Heating / reflux;
|
92.9% |
|
With potassium iodide; calcium chloride; In water; toluene; at 40 - 50 ℃; for 2h; Reagent/catalyst; Solvent;
|
92.2% |
|
5,6-dimethoxy-1-indanone; With tetrabutylammomium bromide; sodium hydroxide; In dichloromethane; water; at 15 - 20 ℃; Large scale;
N-benzyl-4-formylpiperidine; In dichloromethane; water; at 15 - 45 ℃; Large scale;
|
88% |
|
With sodium methylate; In tetrahydrofuran; methanol; at 17 - 43 ℃; for 1h; Product distribution / selectivity;
|
87.6% |
|
With sodium hydroxide; In methanol; toluene; at 65 ℃; for 1h; Product distribution / selectivity; Heating / reflux;
|
84.3% |
|
With sodium hydroxide; In methanol; at 20 ℃; for 3h; Inert atmosphere;
|
84% |
|
With sodium methylate; In methanol; ethanol; toluene; at 79 ℃; for 1h; Product distribution / selectivity; Heating / reflux;
|
82.5% |
|
With sodium methylate; In methanol; propan-1-ol; at 71 - 90 ℃; for 1h; Product distribution / selectivity; Heating / reflux;
|
79.9% |
|
With sodium methylate; In methanol; isopropyl alcohol; at 60 - 80 ℃; for 1h; Product distribution / selectivity; Heating / reflux;
|
78.2% |
|
With sodium methylate; In tetrahydrofuran; methanol; toluene; at 79 ℃; for 1h; Product distribution / selectivity; Heating / reflux;
|
76.4% |
|
With sodium methylate; In methanol; toluene; at 23 - 64 ℃; for 1 - 4.51667h; Product distribution / selectivity;
|
75.4% |
|
With sodium methylate; In methanol; ethyl acetate; at 65 - 71 ℃; for 1.08333h; Product distribution / selectivity; Heating / reflux;
|
75.9% |
|
With sodium methylate; In methanol; isopropyl alcohol; toluene; at 83 ℃; for 1.03333h; Product distribution / selectivity; Heating / reflux;
|
73.1% |
|
With sodium methylate; In methanol; toluene; at 60 - 85 ℃; for 1h; Product distribution / selectivity; Heating / reflux;
|
72.6% |
|
With sodium hydroxide; In methanol; at 20 - 66 ℃; for 6h; Product distribution / selectivity;
|
70% |
|
With sodium methylate; In tetrahydrofuran; methanol; at 63 ℃; for 1h; Product distribution / selectivity; Heating / reflux;
|
69.3% |
|
With sodium methylate; In methanol; propan-1-ol; toluene; at 95 ℃; for 1h; Product distribution / selectivity; Heating / reflux;
|
63.9% |
|
With sodium methylate; In methanol; 1,2-dimethoxyethane; at 60 - 80 ℃; for 1h; Product distribution / selectivity; Heating / reflux;
|
62.9% |
|
With sodium methylate; In methanol; at 20 - 65 ℃; for 2h; Product distribution / selectivity;
|
60% |
|
With N,N,N,N,N,N-hexamethylphosphoric triamide; n-butyllithium; diisopropylamine; Multistep reaction;
|
|
|
5,6-dimethoxy-1-indanone; N-benzyl-4-formylpiperidine; With n-butyllithium; diisopropylamine; In tetrahydrofuran; hexane; at -78 - 20 ℃; for 2.41667h;
In tetrahydrofuran; hexane; at 20 ℃;
|
|
|
With sodium methylate; hydroquinone; In methanol; toluene; at 55 - 70 ℃; for 0.5h;
|
|
|
With sodium hydroxide; In tetrahydrofuran; at 20 - 70 ℃; for 3h;
|
|
|
5,6-dimethoxy-1-indanone; With N,N,N,N,N,N-hexamethylphosphoric triamide; n-butyllithium; diisopropylamine; In tetrahydrofuran; hexane; at -78 ℃; Inert atmosphere;
N-benzyl-4-formylpiperidine; In tetrahydrofuran; hexane; at -78 - 20 ℃; Inert atmosphere;
|
|
|
5,6-dimethoxy-1-indanone; With N,N,N,N,N,N-hexamethylphosphoric triamide; n-butyllithium; diisopropylamine; In tetrahydrofuran; hexane; at -78 - 0 ℃; for 0.416667h;
N-benzyl-4-formylpiperidine; In tetrahydrofuran; hexane; at -78 - 20 ℃; for 2h;
|
|
|
5,6-dimethoxy-1-indanone; With N,N,N,N,N,N-hexamethylphosphoric triamide; n-butyllithium; N-ethyl-N,N-diisopropylamine; In tetrahydrofuran; hexane; at -78 - 0 ℃; for 0.416667h;
N-benzyl-4-formylpiperidine; In tetrahydrofuran; hexane; at 20 ℃; for 2h;
|
|
|
5,6-dimethoxy-1-indanone; N-benzyl-4-formylpiperidine; With potassium hydroxide; In methanol; dichloromethane; at 0 - 30 ℃;
With hydrogenchloride; In methanol; dichloromethane; water; at 25 - 30 ℃; for 0.5h; pH=6.5 - 7.5;
|
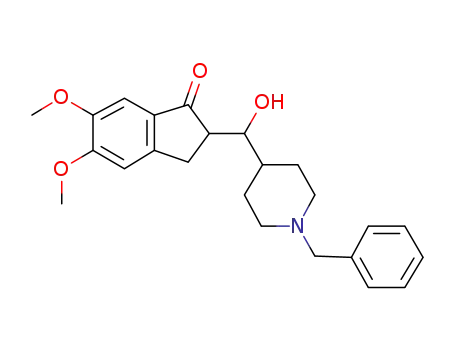
2-((1-benzyl-piperidin-4-yl)-hydroxymethyl)-5,6-dimethoxyindan-1-one

1-benzyl-4-<(5,6-dimethoxy-1-oxoindan-2-ylidenyl)methyl>piperidine
| Conditions | Yield |
|---|---|
|
With alumina solid-supported potassium fluoride; In toluene; at 80 - 100 ℃; for 0.5h; Solvent; Temperature;
|
73.49% |

5,6-dimethoxy-1-indanone

N-benzyl-4-formylpiperidine
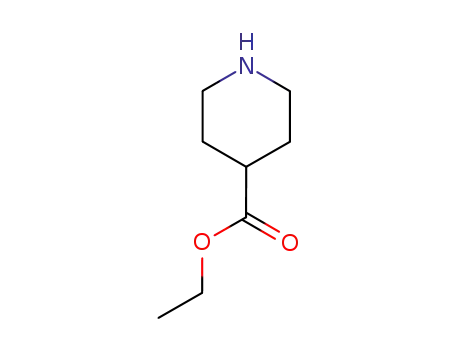
4-carbethoxypiperidine
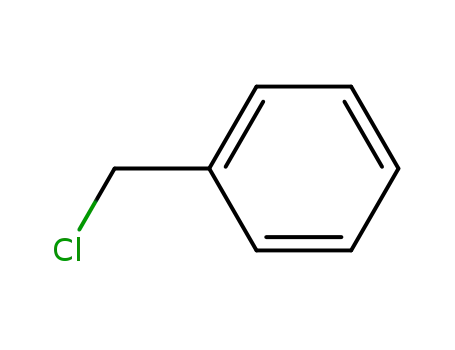
benzyl chloride
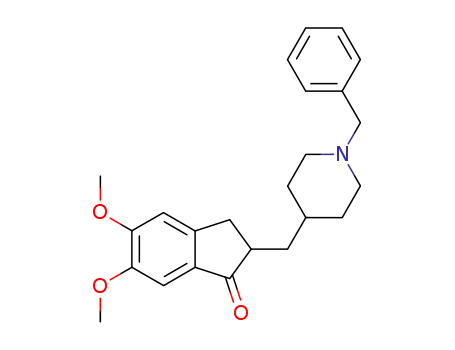
donepezil
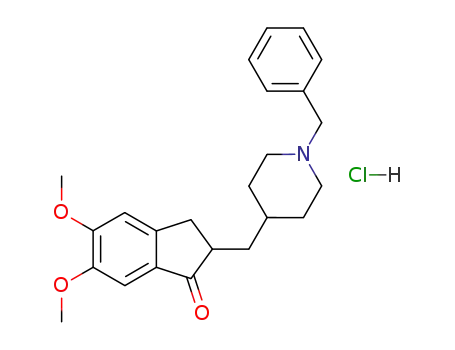
donepezil hydrochloride
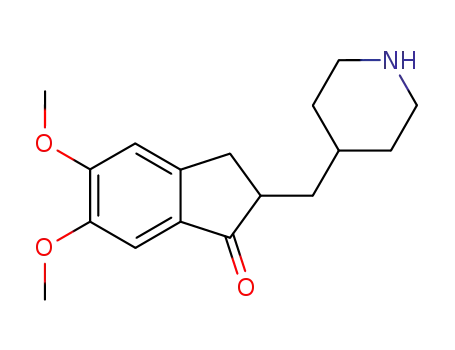
debenzyldonepezil
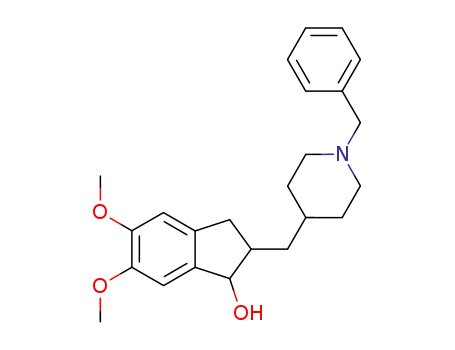
1-benzyl-4-[(5,6-dimethoxy-1-indanol)-2-yl]methylpiperidine
CAS:138071-82-6
CAS:38083-17-9
CAS:22065-85-6
CAS:1187-93-5