- Language:English
- English
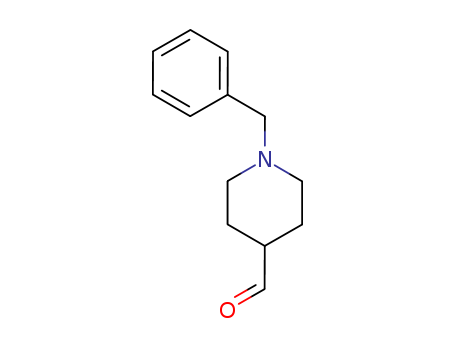

CasNo: 22065-85-6
Molecular Formula: C13H17NO
Appearance: colorless oil
|
Synthesis |
A round bottom flask was charged with oxalyl chloride (16.2 g, 0.12 mol), dichloromethane (150 mL) and anhydrous dimethyl sulfoxide (20 mL). Stir the reaction mixture mass in a cryo bath at -70 °C for 15 min. The resulting mixture is charged dropwise with N-benzyl piperidine alcohol 3 (20 g, 0.097 mol), along with triethylamine (24.6 g, 0.24 mol) and continued stirring for the next 15 min. After that, the mass is allowed to attain room temperature overnight, then diluted with dichloromethane (100 mL) and quenched with cold water. The organic layer was washed subsequently with 5 % HCl solution, brine solution, 5 % sodium bicarbonate solution and dried over sodium sulfate. Toluene was removed in vacuo to afford N-Benzylpiperidine-4-carboxaldehyde (19.2 g, 96 %). |
|
Chemical Properties |
Colorless Oil |
|
Uses |
Reactant for:Stereospecific allylic alkylationReactions of Grignard reagents with carbonyl compoundsFluorodenitrations and nitrodehalogenations for labeled PET ligands and fluoropharmaceuticalsSelective α1 receptor antagonistsReactant for synthesis of:DonepezilMCH-R1 antagonists |
IUPAC Name: 1-benzylpiperidine-4-carbaldehyde
Isomeric SMILES: C1CN(CCC1C=O)CC2=CC=CC=C2
InChIKey: SGIBOXBBPQRZDM-UHFFFAOYSA-N
InChI: InChI=1S/C13H17NO/c15-11-13-6-8-14(9-7-13)10-12-4-2-1-3-5-12/h1-5,11,13H,6-10H2
Alzheimer’s disease (AD) is a multifacto...
A new route towards the synthesis of N-s...
… available N-benzylpiperidine-4-carboxaldehyde 10 was … the synthesis of N-benzylpiperidine-4-carboxaldehyde 12 from … nucleus and N-benzylpiperidine-4-carboxaldehyde derivatives …
The iron storage protein bacterioferriti...
1-benzylpiperidine-4-carboxylic acid methyl ester

N-benzyl-4-formylpiperidine
| Conditions | Yield |
|---|---|
|
With red-aluminum/morpholine complex; In toluene; at -5 - 0 ℃; for 0.5h; Reagent/catalyst; Solvent; Inert atmosphere; Large scale;
|
96.5% |
|
Multi-step reaction with 2 steps
1: 69.5 percent / LiAlH4 / tetrahydrofuran / 0.5 h / 50 °C
2: 94.3 percent / oxalyl chloride; dimethyl sulfoxide; Et3N / CH2Cl2 / 0.25 h / -55 °C
With lithium aluminium tetrahydride; oxalyl dichloride; dimethyl sulfoxide; triethylamine; In tetrahydrofuran; dichloromethane; 1: Reduction / 2: Swern oxidation;
|
|
|
Multi-step reaction with 4 steps
1.1: water; methanol; sodium hydroxide / 2 h / Reflux
2.1: thionyl chloride / 2 h / Reflux
2.2: 0.5 h / Cooling with ice
3.1: thionyl chloride / 5 h / Reflux
4.1: diisobutylaluminium hydride / toluene / 1 h / 0 °C
4.2: 0.5 h / pH 8
With methanol; thionyl chloride; water; diisobutylaluminium hydride; sodium hydroxide; In toluene;
|
1-benzylpiperidine-4-carbonitrile


N-benzyl-4-formylpiperidine
| Conditions | Yield |
|---|---|
|
1-benzylpiperidine-4-carbonitrile; With diisobutylaluminium hydride; In toluene; at 0 ℃; for 1h;
With water; sodium hydroxide; In methanol; toluene; for 0.5h; pH=8; Temperature;
|
97.5% |
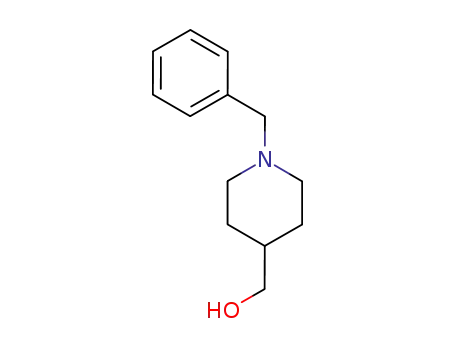
1-benzyl-4-piperidinemethanol
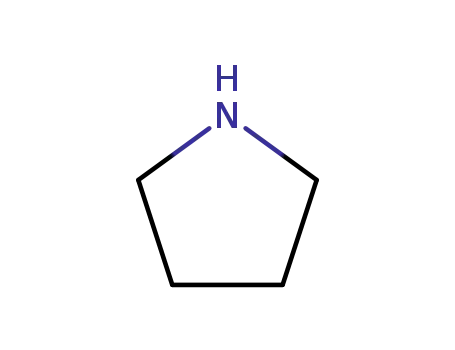
pyrrolidine
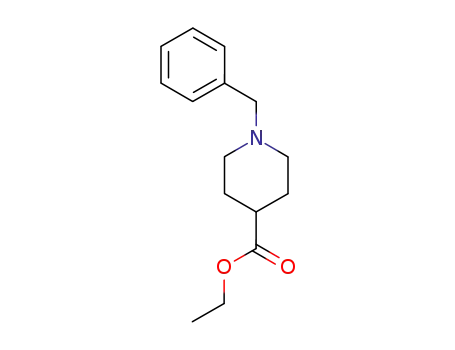
N-benzyl isonipecotic acid ethyl ester
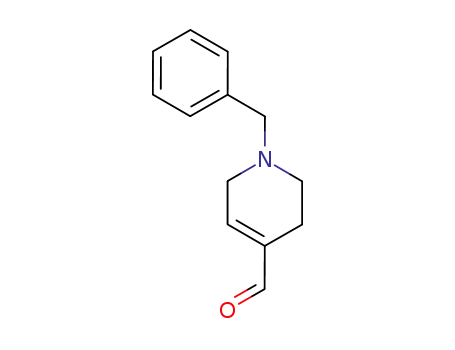
1-benzyl-1,2,3,6-tetrahydropyridine-4-carbaldehyde
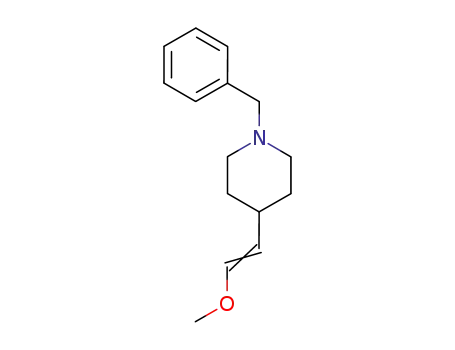
1-benzyl-4-(methoxyethenyl)piperidine
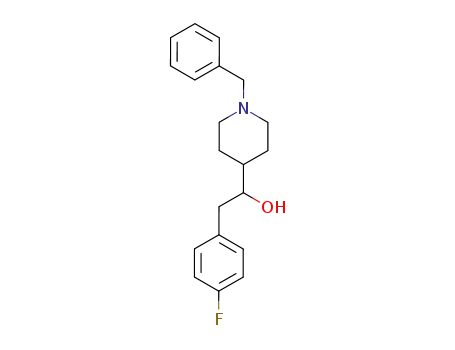
1-benzyl-4-<2-(4-fluorophenyl)-1-hydroxyethyl>piperidine
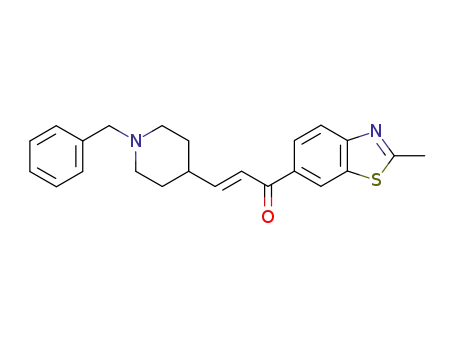
1-(2-methyl-6-benzothiazolyl)-3-(N-benzyl-4-piperidinyl)-2-propen-1-one
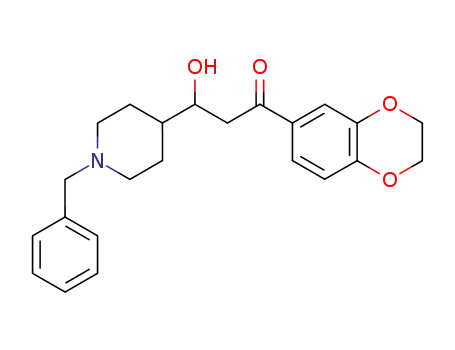
3-(1-benzylpiperidin-4-yl)-1-(2,3-dihydrobenzo<1,4>dioxin-6-yl)-3-hydroxypropan-1-one
CAS:138071-82-6
CAS:38083-17-9
CAS:120014-06-4
CAS:120014-07-5