- Language:English
- English
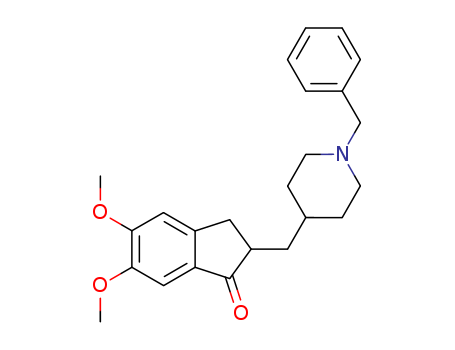

CasNo: 120014-06-4
Molecular Formula: C24H29NO3
Appearance: white or almost white crystal powder
|
Description |
Donepezil is a specific and potent acetylcholinesterase inhibitor according to in vitro data. It displays primarily noncompetitive inhibitory activity. Donepezil is another “nonclassic,” centrally acting, reversible, noncompetitive AChEI that was approved in 1997 for treatment of mild-to-moderate AD and dementia. Its selectivity for AChE is 570- to 1,250-fold that for butyrylcholinesterase, and it also exhibits greater affinity for brain AChE than for AChE in the periphery. 2-[(1-Benzyl-4-piperidyl)methyl]-5,6-dimethoxy-2,3-dihydroinden-1-one is a chemical compound that is not approved for use in humans. Therefore, its safety and efficacy have not been established. It is important to note that the safety of any chemical compound depends on various factors, including the dosage, route of administration, and individual factors such as age, health status, and medical history. |
|
Uses |
Donepezil works by increasing the levels of a neurotransmitter called acetylcholine in the brain, which can help improve cognitive function and memory. Donepezil is available in various forms, including tablets and orally disintegrating tablets, and is typically taken once daily. As with any medication, it is important to follow your doctor's instructions carefully and to report any side effects or concerns. |
|
Definition |
ChEBI: Donepezil is a centrally acting reversible acetyl cholinesterase inhibitor. Its main therapeutic use is in the treatment of Alzheimer's disease where it is used to increase cortical acetylcholine. |
|
Brand name |
Aricept (Eisai Medical Research). |
Isomeric SMILES: COC1=C(C=C2C(=C1)CC(C2=O)CC3CCN(CC3)CC4=CC=CC=C4)OC
InChIKey: ADEBPBSSDYVVLD-UHFFFAOYSA-N
InChI: InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3
The relative in vitro skin permeation rate of donepezil (DP) through the hairless mouse skin showed a parabolic relationship with increased carbon length of the fatty acid enhancers.
Our main analysis compared the safety and efficacy of donepezil 10 mg/day with placebo at 24 to 26 weeks of treatment. This can be done by a group of drugs known as cholinesterase inhibitors. Donepezil is a cholinesterase inhibitor.
A new, economical, and efficient process...
Highly valued products resulting from re...
A facile method for the synthesis of ind...
Hydrogenation of over a dozen aromatic c...
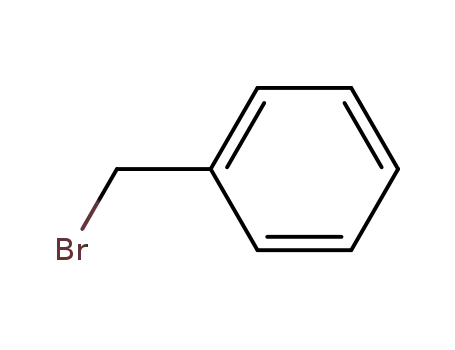
benzyl bromide

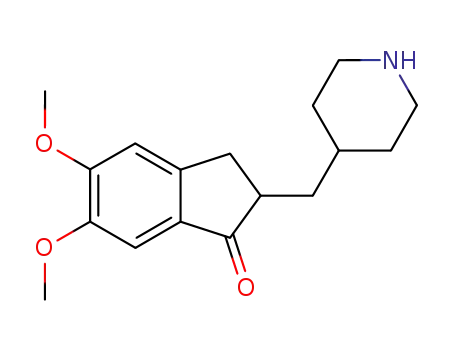
debenzyldonepezil


donepezil
| Conditions | Yield |
|---|---|
|
With sodium carbonate; In ethanol; at 50 - 60 ℃; for 6h;
|
92.3% |
|
With triethylamine; In dichloromethane; for 4h; Heating / reflux;
|
|
|
benzyl bromide; debenzyldonepezil; With tetrabutylammomium bromide; potassium carbonate; In dichloromethane; water; at 20 ℃;
With hydrogenchloride; In methanol;
|
|
|
With sodium carbonate; In methanol; isopropyl alcohol; at 55 - 60 ℃; for 11h;
|
|
|
benzyl bromide; debenzyldonepezil; With potassium carbonate; acetic acid; palladium 10% on activated carbon; In water; ethyl acetate; at 25 - 30 ℃; for 4h;
With sodium hydroxide; In water; ethyl acetate;
|
|
|
With sodium carbonate; In isopropyl alcohol; at 50 ℃; for 12h;
|
32.3 mg |
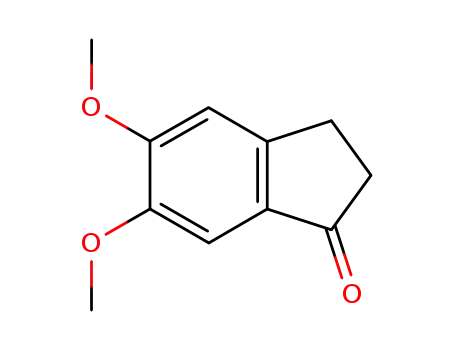
5,6-dimethoxy-1-indanone

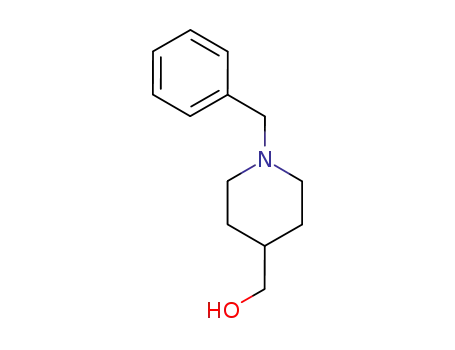
1-benzyl-4-piperidinemethanol


donepezil
| Conditions | Yield |
|---|---|
|
With bis(μ-chloro)-bis[1,3-di(2-pyridyl)-4,6-dimethylbenzene-N,C(2'),N-iridium chloride]; caesium carbonate; In tert-Amyl alcohol; for 12h; Reflux;
|
85% |
|
With [Ir(dpyx-N,C,N)Cl(i-Cl)]2; caesium carbonate; In tert-Amyl alcohol; at 130 ℃; for 2h; Reagent/catalyst;
|
85% |
|
With potassium phosphate tribasic trihydrate; C39H32Cl2N5PRu; In tert-Amyl alcohol; at 120 ℃; for 4h; Inert atmosphere; Schlenk technique;
|
83% |
|
With C39H32Cl2N5PRu; potassium tert-butylate; In tert-Amyl alcohol; at 120 ℃; for 12h; Solvent; Reagent/catalyst; Inert atmosphere; Schlenk technique;
|
83% |
|
With [(Cp*IrCl)2(4,4′,6,6′-tetrahydroxy-2,2′-bipyrimidine)][Cl]2; potassium hydroxide; In water; at 100 ℃; for 12h; Green chemistry;
|
82% |
|
With [(Cp*IrCl)2(4,4′,6,6′-tetrahydroxy-2,2′-bipyrimidine)][Cl]2; potassium hydroxide; In water; at 130 ℃; for 12h; Reagent/catalyst;
|
82% |
|
With trifuran-2-yl-phosphane; C48H34F6N4O4Pd2; lithium hydroxide; In neat (no solvent); at 100 ℃; for 48h; Inert atmosphere; Schlenk technique; Sealed tube;
|
46% |
|
With 1,10-Phenanthroline; potassium tert-butylate; nickel dibromide; In toluene; at 140 ℃; for 36h; Schlenk technique; Sealed tube; Inert atmosphere;
|
46% |
|
With [bis(2-methylallyl)cycloocta-1,5-diene]ruthenium(II); 1,3-bis((R)-1-(naphthalen-1-yl)ethyl)-4,5-dihydro-1H-imidazol-3-ium tetrafluoroborate; potassium tert-butylate; lithium tert-butoxide; In hexane; pentan-1-ol; at 100 ℃; for 24h;
|
40% |
|
With 1,10-Phenanthroline; bis(acetylacetonato)manganese(II); potassium tert-butylate; In toluene; at 140 ℃; for 36h; Schlenk technique; Inert atmosphere;
|
40% |
|
With diiron nonacarbonyl; potassium tert-butylate; In toluene; at 140 ℃; for 24h; Schlenk technique; Inert atmosphere;
|
36% |
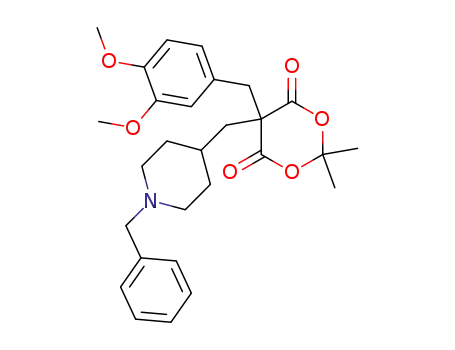
5-[(1-benzyl-4-piperidyl)methyl]-5-(3,4-dimethoxybenzyl)-2,2-dimethyl-1,3-dioxane-4,6-dione
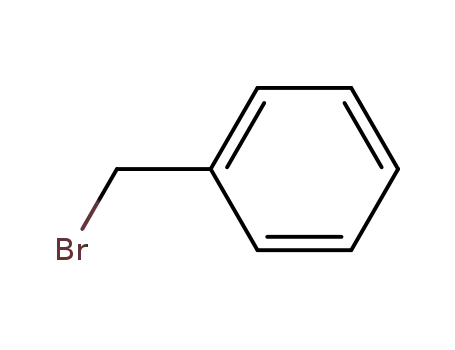
benzyl bromide

debenzyldonepezil
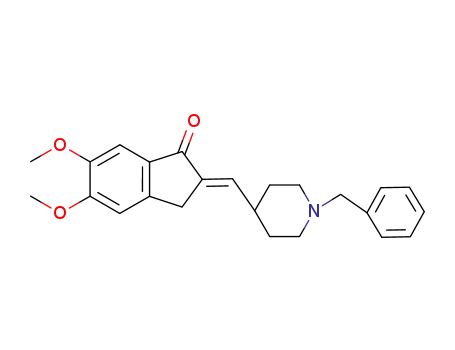
(E)-2-((1-benzylpiperidine-4-yl)methylene)-5,6-dimethoxy-2,3-dihydro-1H-inden-1-one
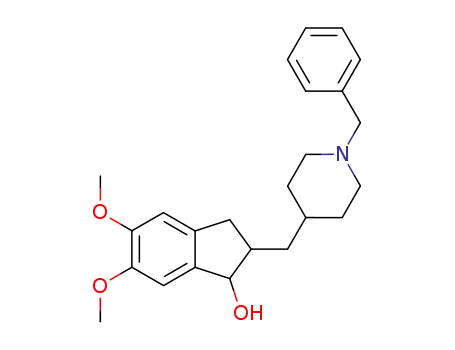
1-benzyl-4-[(5,6-dimethoxy-1-indanol)-2-yl]methylpiperidine
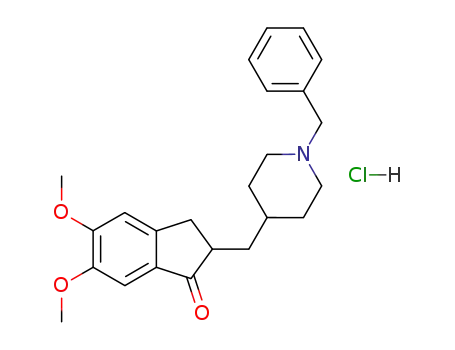
donepezil hydrochloride
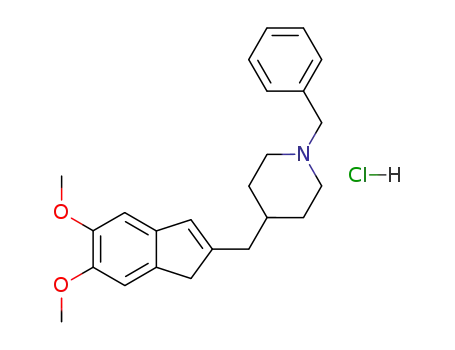
1-Benzyl-4-[(5,6-dimethoxyinden)-2-yl]methylpiperidine hydrochloride
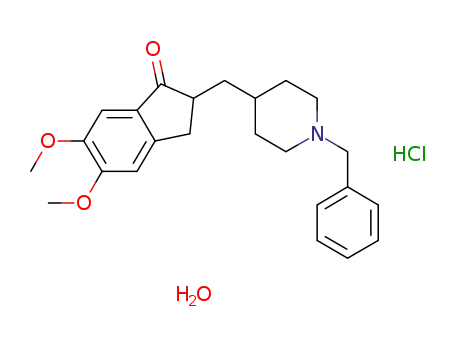
1-benzyl-4-((5,6-dimethoxy-1-indanon)-2-yl)methylpiperidine hydrochloride monohydrate
CAS:138071-82-6
CAS:38083-17-9
CAS:120014-30-4
CAS:22065-85-6