- Language:English
- English
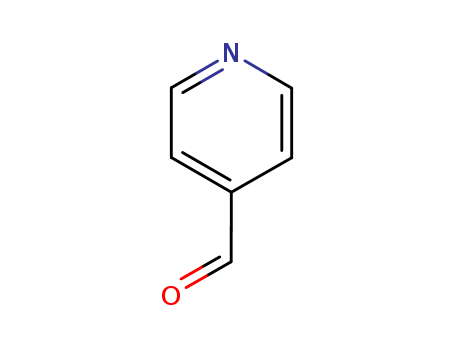

CasNo: 872-85-5
Molecular Formula: C6H5NO
Appearance: clear yellow-brown liquid
|
Flavor Type |
Flavor Type: fruity |
|
Chemical Properties |
4-Pyridinecarboxaldehyde(4PCA) is a slightly yellow oily liquid. Its relative density is 1.122, and its refractive index is 1.5352 (25°C). Flash point is 54 ° C. 4-Pyridinecarboxaldehyde is soluble in water and ether.4-Pyridinecarboxaldehyde, also commonly called pyridine-2-carboxaldehyde, is an organic compound with the formula NC5H4CHO. 4-Pyridinecarboxaldehyde is a colorless oily liquid with a distinctive odor. Older samples are often brown-colored owing to impurities. |
|
Uses |
4-Pyridinecarboxaldehyde is an heterocyclic building block used for the synthesis of various pharmaceutical compounds, such as new 1,4-dihydropyridin-4-yl-phenoxyacetohydrazones, having anticonvulsant and anti-inflammatory properties.4-Pyridinecarboxaldehyde and some of its derivatives have also been reported as useful transamination reagents to introduce ketone or aldehyde groups onto the N-termini of antibodies for subsequent site-specifically conjugate aminooxy-functionalized molecules (including fluorescent dyes, polyethylene glycol, or porphyrins) to these entities. 4-Pyridinecarboxaldehyde can be used for the synthesis of: β-Unsaturated amides by coupling with N,N-disubstituted formamides; meso-Substituted A3-corroles; N-(4-pyridylmethyl)-L-valine as a ligand to construct zinc metal–organic frameworks (Zn-MOFs); 4′-Pyridyl terpyridines, with potential application as anticancer and antimicrobial agents; 4-pyridinecarboxaldehyde thiosemicarbazone, as a corrosion inhibitor for mild steel. |
|
Preparation |
4-Pyridinecarbaldehyde is synthesized by oxidation of 4-picoline. The mixed gas of 4-picoline and air is passed through the vanadium-molybdenum catalyst layer heated to 400°C, and oxidized to generate 4-pyridinecarbaldehyde. |
|
Synthesis Reference(s) |
Synthetic Communications, 20, p. 3385, 1990 DOI: 10.1080/00397919008051576 |
|
Purification Methods |
Purified as for pyridine-2-aldehyde. [Beilstein 21 III/IV 2529, 21/7 V 351.] |
InChI:InChI=1/C6H5NO/c8-5-6-1-3-7-4-2-6/h1-5H
Remarkable electrocatalytic property of ...
Herein, a well-defined nanostructure wit...
Bifunctional catalysts have been conside...
Catalytic oxidation of alcohols containi...
Grignard-type additions were readily ach...
-
We have reported an aerobic oxidation of...
A novel strategy combining visible-light...
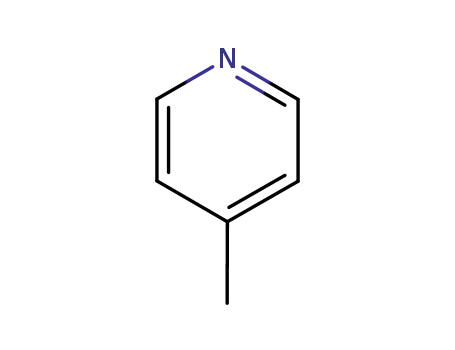
picoline

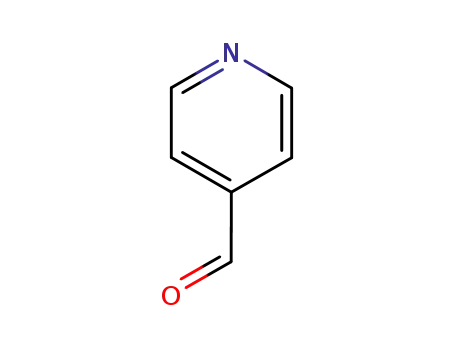
pyridine-4-carbaldehyde

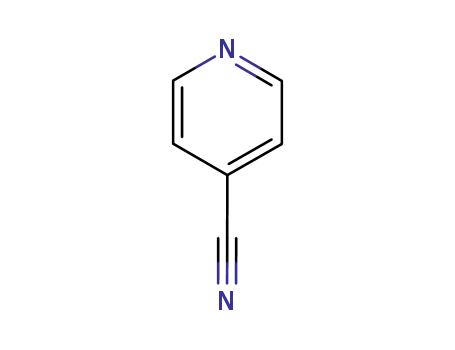
pyridine-4-carbonitrile

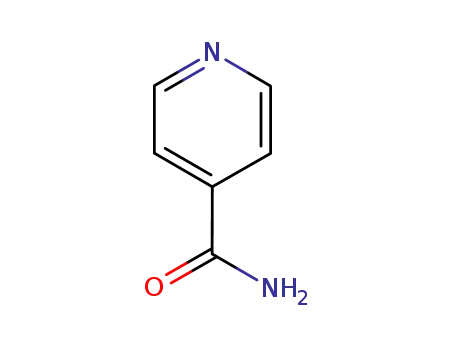
isonicotinamide
| Conditions | Yield |
|---|---|
|
With
manganese(IV) oxide; oxygen; urea;
at 150 ℃;
for 3h;
under 3800.26 Torr;
Autoclave;
|
78 %Chromat. 8 %Chromat. 52 %Chromat. |

picoline

![2,2'-diphenyl-[3,3']biindolylidene 1,1'-dioxide](/upload/2023/6/2a578130-bbf7-4cae-8e48-7ed34b0e9c57.png)
2,2'-diphenyl-[3,3']biindolylidene 1,1'-dioxide

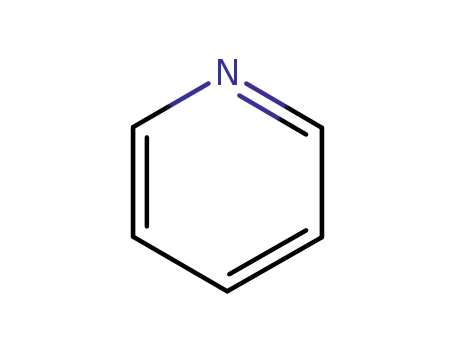
pyridine


pyridine-4-carbaldehyde

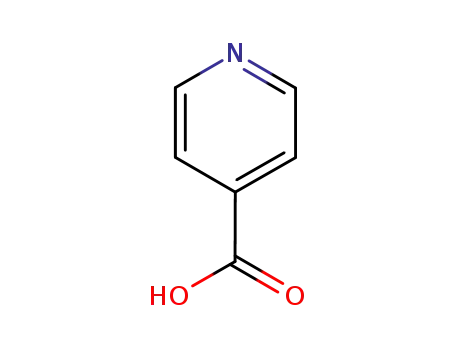
pyridine-4-carboxylic acid

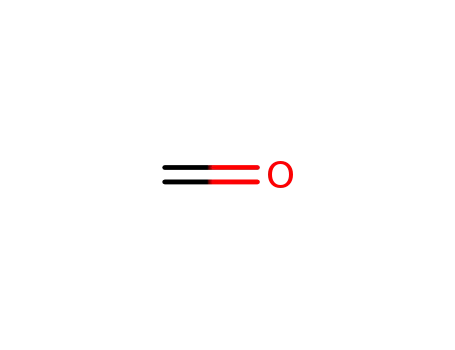
formaldehyd

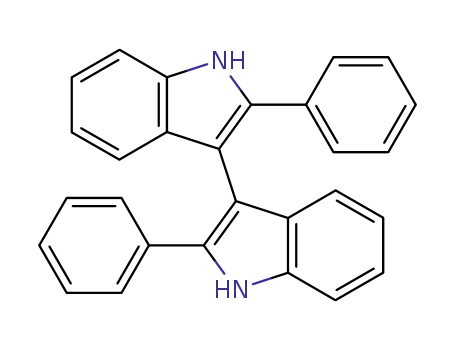
2,2'-diphenyl-1H,1'H-3,3'-biindole
| Conditions | Yield |
|---|---|
|
at 140 ℃;
for 14h;
Product distribution;
|
55% 13% 10% 31% |

picoline

pyridine-4-carbonitrile
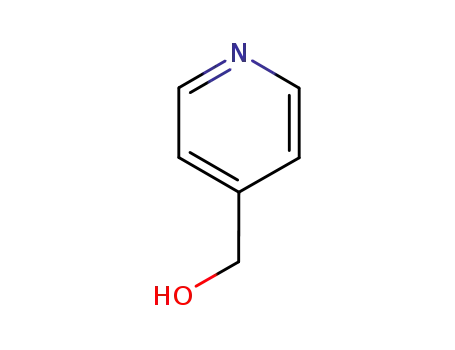
pyridine-4-methanol
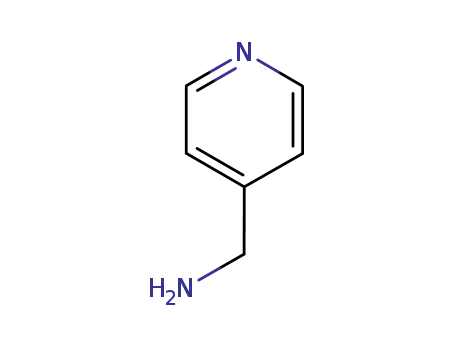
4-(aminomethyl)pyridine
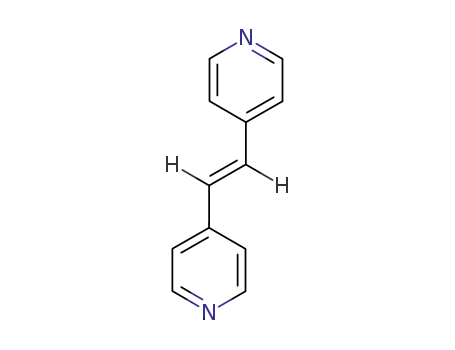
trans-1,2-bis(pyridin-4-yl)ethene
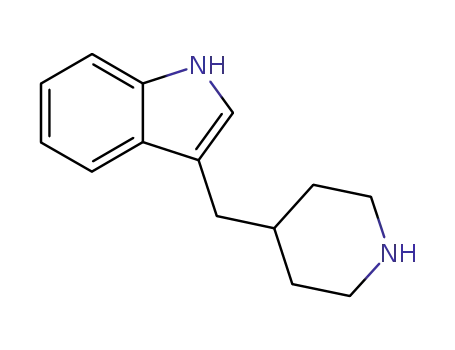
3-<4>Piperidylmethyl-indol
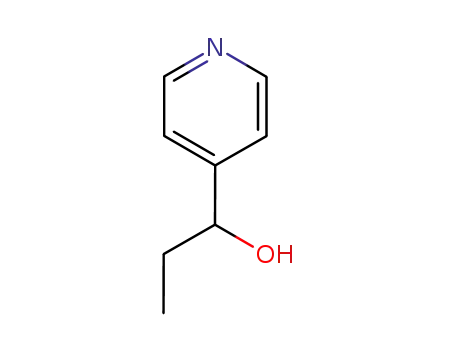
4-hydroxypropylpyridine
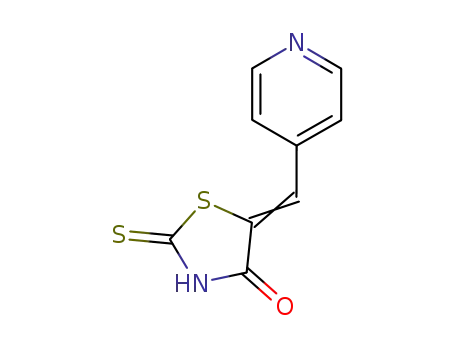
NL-19
CAS:138071-82-6
CAS:38083-17-9
CAS:2107-69-9
CAS:120014-30-4