- Language:English
- English
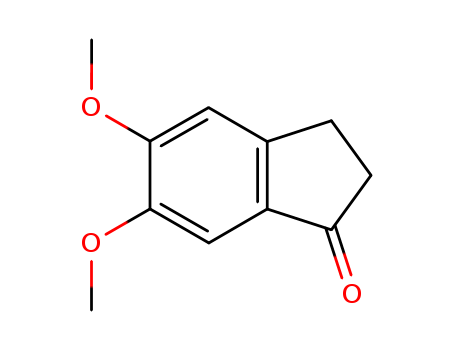

CasNo: 2107-69-9
Molecular Formula: C11H12O3
Appearance: white to light yellow crystal powder
|
Chemical Properties |
white to light yellow crystal powder |
|
Uses |
5,6-Dimethoxy-1-indanone can be an intermediate useful in the preparation of Donepezil. |
|
General Description |
Thiosemicarbazone derived from 5,6-dimethoxy-1-indanone inhibits bovine viral diarrhea virus infection. |
|
Purification Methods |
Crystallise the indanone from MeOH, then sublime it in a vacuum. [Beilstein 8 IV 1985.] |
InChI:InChI=1/C9H10N2O2/c1-12-8-3-6-7(11-5-10-6)4-9(8)13-2/h3-5H,1-2H3,(H,10,11)
-
A green, efficient and cheap demethylati...
A facile method for the synthesis of ind...
In this paper, a novel and open-vessel r...
A series of 1-trifluoromethyl substitute...
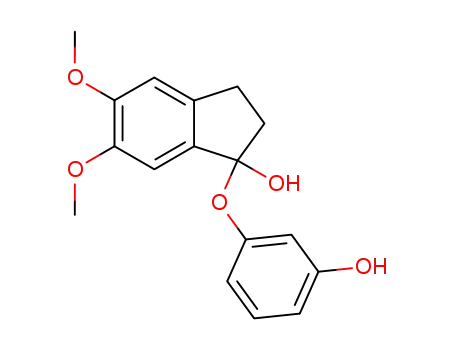
1-(3-Hydroxy-phenoxy)-5,6-dimethoxy-indan-1-ol

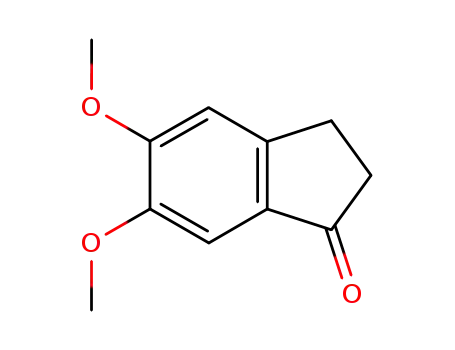
5,6-dimethoxy-1-indanone

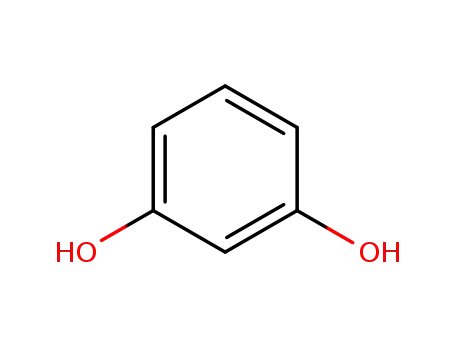
recorcinol
| Conditions | Yield |
|---|---|
|
|
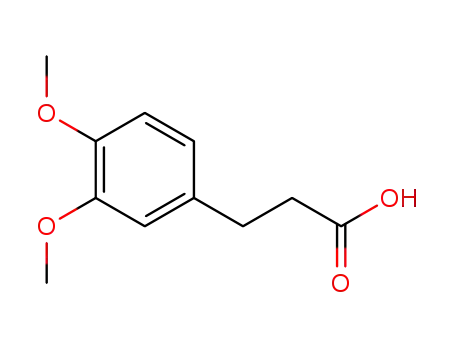
3,4-methoxycinnamic acid


5,6-dimethoxy-1-indanone
| Conditions | Yield |
|---|---|
|
With
trifluorormethanesulfonic acid;
In
dichloromethane;
at 80 ℃;
for 1h;
High pressure;
Inert atmosphere;
Green chemistry;
|
100% |
|
With
trifluorormethanesulfonic acid;
In
dichloromethane;
at 0 - 80 ℃;
|
100% |
|
With
trifluorormethanesulfonic acid;
In
dichloromethane;
at 0 - 80 ℃;
for 1.5h;
Sealed tube;
|
96% |
|
With
methanesulfonic acid; phosphorus pentoxide;
at 100 ℃;
for 0.05h;
|
95% |
|
With
phosphorus pentoxide; phosphoric acid;
at 40 - 60 ℃;
Product distribution / selectivity;
Inert atmosphere;
|
87% |
|
With
phosphorus pentoxide; toluene-4-sulfonic acid;
at 120 ℃;
for 0.0833333h;
|
87% |
|
3,4-methoxycinnamic acid;
With
thionyl chloride; N,N-dimethyl-formamide;
In
dichloromethane;
Reflux;
With
aluminum (III) chloride;
In
dichloromethane;
at 0 - 20 ℃;
for 1.5h;
|
85% |
|
3,4-methoxycinnamic acid;
With
trifluoroacetic anhydride;
In
acetonitrile;
at 25 ℃;
for 0.166667h;
With
3,4-dimethoxy-benzaldehyde;
In
acetonitrile;
at 25 ℃;
for 10h;
|
80% |
|
With
trifluorormethanesulfonic acid;
In
dichloromethane;
at 80 ℃;
for 1h;
|
78% |
|
With
methanesulfonic acid; phosphorus pentoxide;
at 100 ℃;
for 0.0833333h;
|
74% |
|
With
phosphorus pentoxide; phosphoric acid;
at 120 ℃;
for 6h;
|
55% |
|
3,4-methoxycinnamic acid;
With
oxalyl dichloride;
In
dichloromethane; N,N-dimethyl-formamide;
at 20 ℃;
for 18h;
With
aluminum (III) chloride;
In
dichloromethane;
at 20 ℃;
for 2h;
|
34% |
|
With
phosphorus pentachloride;
Behandeln des Reaktionsprodukts in Petrolaether mit AlCl3;
|
|
|
With
phosphorus pentoxide; benzene;
|
|
|
With
hydrogen fluoride;
|
|
|
With
water; phosphorus pentoxide; benzene;
|
|
|
With
phosphoric acid; phosphorus pentoxide;
|
|
|
With
PPA;
|
|
|
With
phosphorus pentaoxide;
In
benzene;
|
|
|
Multi-step reaction with 2 steps
1: oxalyl dichloride / dichloromethane; N,N-dimethyl-formamide / 20 °C
2: aluminum (III) chloride / dichloromethane / 2 h / 20 °C
With
aluminum (III) chloride; oxalyl dichloride;
In
dichloromethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1: dicyclohexyl-carbodiimide; dmap / dichloromethane / 0 - 20 °C / Inert atmosphere
2: aluminum (III) chloride / dichloromethane / 0 - 20 °C / Inert atmosphere
With
dmap; aluminum (III) chloride; dicyclohexyl-carbodiimide;
In
dichloromethane;
2: |Friedel-Crafts Acylation;
|
|
|
Multi-step reaction with 2 steps
1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 20 °C / Inert atmosphere
2: aluminum (III) chloride / dichloromethane / 2 h / 0 - 20 °C / Inert atmosphere
With
aluminum (III) chloride; oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
|
|
|
With
polyphosphoric acid;
at 20 - 100 ℃;
for 2h;
|
|
|
Multi-step reaction with 2 steps
1: N,N-dimethyl-formamide; oxalyl dichloride / dichloromethane / 8 h / 20 °C
2: aluminum (III) chloride / dichloromethane / 3.5 h / 0 - 20 °C
With
aluminum (III) chloride; oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: oxalyl dichloride / dichloromethane; N,N-dimethyl-formamide / 8 h / 20 °C
2: aluminum (III) chloride / dichloromethane / 3.5 h / 0 - 20 °C
With
aluminum (III) chloride; oxalyl dichloride;
In
dichloromethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1: N,N-dimethyl-formamide; oxalyl dichloride / dichloromethane / 12 h / 20 °C
2: aluminum (III) chloride / dichloromethane / 2.5 h / 0 - 20 °C
With
aluminum (III) chloride; oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
2: |Friedel-Crafts Acylation;
|
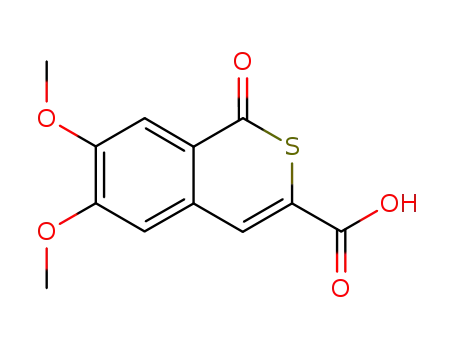
6,7-dimethoxy-1-oxo-1H-isothiochromene-3-carboxylic acid

3,4-methoxycinnamic acid
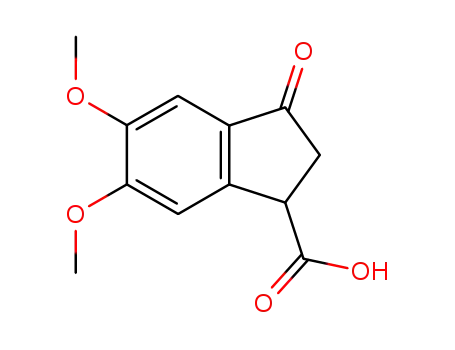
5,6-dimethoxy-3-oxo-indan-1-carboxylic acid
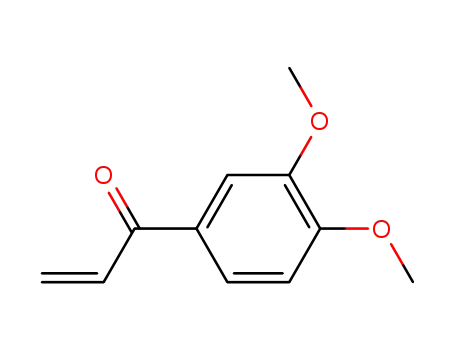
1-(3,4-dimethoxyphenyl)prop-2-en-1-one
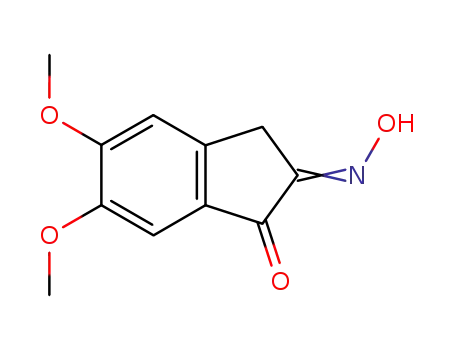
5,6-dimethoxy-indan-1,2-dione-2-oxime
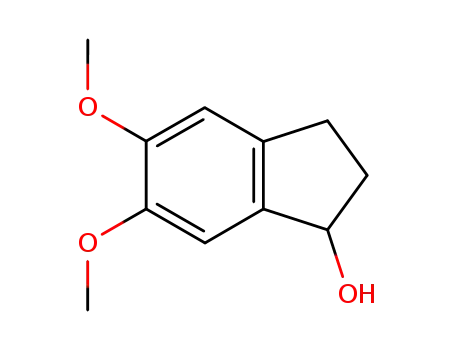
5,6-dimethoxy-2,3-dihydro-1H-inden-1-ol
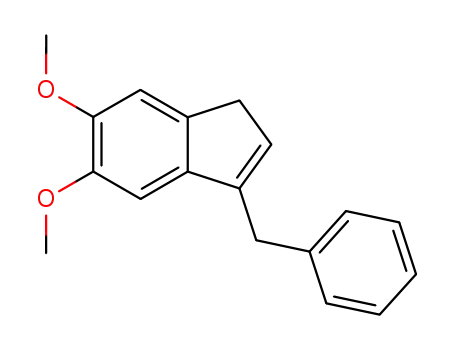
3-Benzyl-5,6-dimethoxy-1H-indene
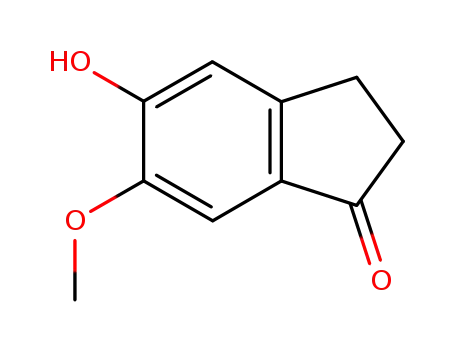
5-hydroxy-6-methoxy-2,3-dihydro-1H-inden-1-one
CAS:138071-82-6
CAS:57280-22-5
CAS:120013-39-0
CAS:872-85-5