- Language:English
- English


CasNo: 817204-32-3
Molecular Formula: C31H26FN3O7
|
Uses |
(2'R)-N-Benzoyl-2'-deoxy-2'-fluoro-2'-methylcytidine -3',5'-dibenzoate is an impurity of Sofosbuvir (P839640). To support clinical development efforts, we needed an efficient and scalable synthesis of PSI-6130. |
IUPAC Name: [(2R,3R,4R,5R)-5-(4-benzamido-2-oxopyrimidin-1-yl)-3-benzoyloxy-4-fluoro-4-methyloxolan-2-yl]methyl benzoate
Isomeric SMILES: C[C@]1([C@@H]([C@H](O[C@H]1N2C=CC(=NC2=O)NC(=O)C3=CC=CC=C3)COC(=O)C4=CC=CC=C4)OC(=O)C5=CC=CC=C5)F
InChIKey: MXEQSUUFNWPUJH-RDWHIKKYSA-N
InChI: InChI=1S/C31H26FN3O7/c1-31(32)25(42-28(38)22-15-9-4-10-16-22)23(19-40-27(37)21-13-7-3-8-14-21)41-29(31)35-18-17-24(34-30(35)39)33-26(36)20-11-5-2-6-12-20/h2-18,23,25,29H,19H2,1H3,(H,33,34,36,39)/t23-,25-,29-,31-/m1/s1
Sofosbuvir (vide supra) has recently been approved as a therapeutic agent for the treatment of hepatitis C. This prodrug is synthesized via the late-stage nucleoside intermediate PSI-6130 (817204-32-3), which we hypothesized could be accessed rapidly using our pentose strategy.
The invention discloses a process method...
The invention provides a method for impr...
Through systematical comparison of vario...
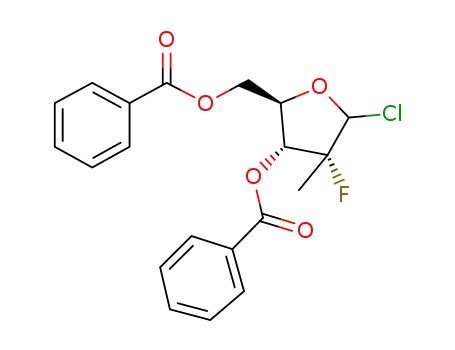
(2R)-2-deoxy-2-fluoro-2-methyl-α/β-D-erythro-pentofuranosyl chloride-3,5-dibenzoate

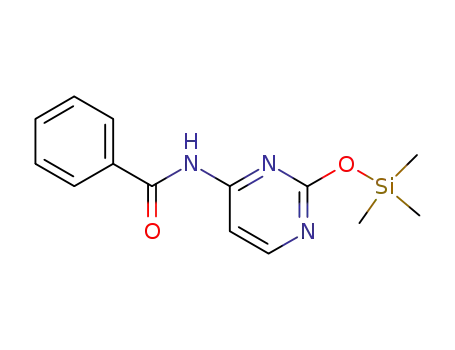
N-(2-Trimethylsilanyloxy-pyrimidin-4-yl)-benzamide

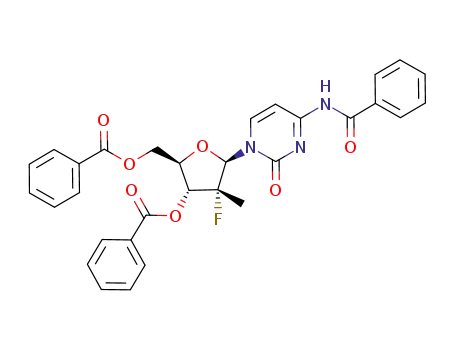
(2R,3R,4R,5R)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4-fluoro-4-methyltetrahydrofuran-3-yl benzoate
| Conditions | Yield |
|---|---|
|
(2R)-2-deoxy-2-fluoro-2-methyl-α/β-D-erythro-pentofuranosyl chloride-3,5-dibenzoate; N-(2-Trimethylsilanyloxy-pyrimidin-4-yl)-benzamide; In chlorobenzene; at 23 ℃; for 0.25h;
With tin(IV) chloride; In chlorobenzene; at 50 - 60 ℃; for 18h;
|
72.5% |
|
With tin(IV) chloride; In chlorobenzene; at 75 ℃;
|
65% |
|
(2R)-2-deoxy-2-fluoro-2-methyl-α/β-D-erythro-pentofuranosyl chloride-3,5-dibenzoate; N-(2-Trimethylsilanyloxy-pyrimidin-4-yl)-benzamide; With tin(IV) chloride; In chlorobenzene; at 70 ℃; for 10h;
With water; sodium hydrogencarbonate; In dichloromethane; chlorobenzene; at 10 - 45 ℃; for 0.5h;
|
|
|
(2R)-2-deoxy-2-fluoro-2-methyl-α/β-D-erythro-pentofuranosyl chloride-3,5-dibenzoate; N-(2-Trimethylsilanyloxy-pyrimidin-4-yl)-benzamide; With tin(IV) chloride; In dichloromethane; at 30 - 80 ℃; for 20h; under 1875.19 Torr; Sealed tube;
With acetic acid; In dichloromethane; water; at 18 - 25 ℃;
|
34.9 g |
|
(2R)-2-deoxy-2-fluoro-2-methyl-α/β-D-erythro-pentofuranosyl chloride-3,5-dibenzoate; N-(2-Trimethylsilanyloxy-pyrimidin-4-yl)-benzamide; With tin(IV) chloride; In dichloromethane; at 75 - 80 ℃; for 20h; under 1875.19 Torr;
With acetic acid; In dichloromethane; water; at 18 - 25 ℃;
|
34.9 g |
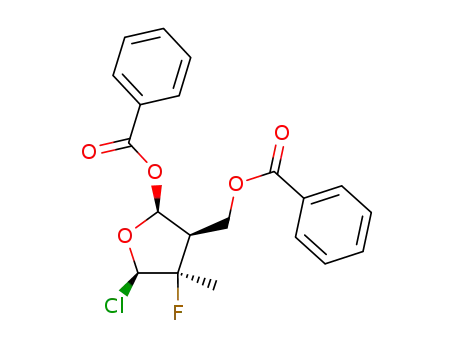
((2R,3R,4R,5R)-3-(benzoyloxy)-5-chloro-4-fluoro-4-methyltetrahydrofuran-2-yl)methylbenzoate


N-(2-Trimethylsilanyloxy-pyrimidin-4-yl)-benzamide


(2R,3R,4R,5R)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4-fluoro-4-methyltetrahydrofuran-3-yl benzoate
| Conditions | Yield |
|---|---|
|
With tin(IV) chloride; acetic acid; In dichloromethane; chlorobenzene; at 70 - 85 ℃; for 0.0166667h; under 750.075 - 2250.23 Torr; Reagent/catalyst; Temperature; Pressure;
|
72% |
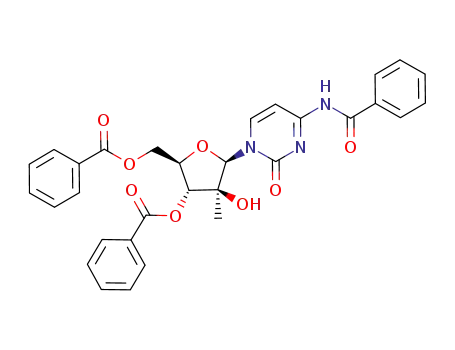
N4-benzoyl-1-(2-C-methyl-3,5-di-O-benzoyl-β-D-arabinofuranosyl)cytosine
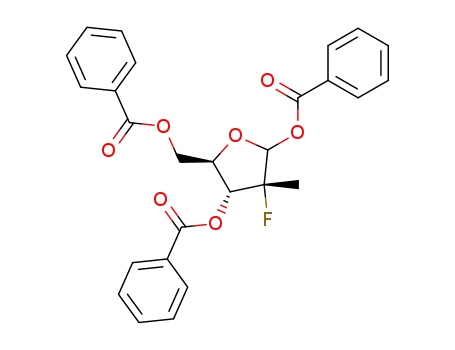
1,3,5-tri-O-benzoyl-2-deoxy-2-fluoro-2-C-methyl-D-ribofuranose

(2R)-2-deoxy-2-fluoro-2-methyl-α/β-D-erythro-pentofuranosyl chloride-3,5-dibenzoate

N-(2-Trimethylsilanyloxy-pyrimidin-4-yl)-benzamide
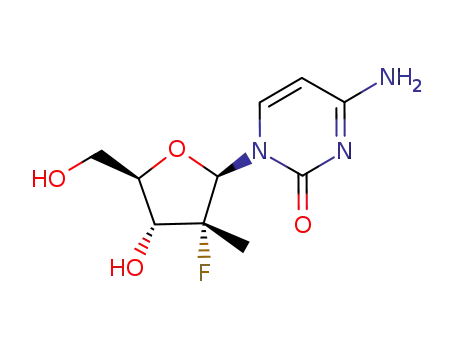
(2'R)-2'-deoxy-2'-fluoro-2'-methylcytidine
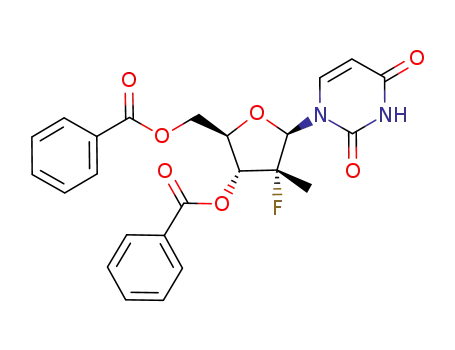
(2‘R)-2‘-deoxy-2’-fluoro-2-methyluridine-3’,5’-dibenzoate
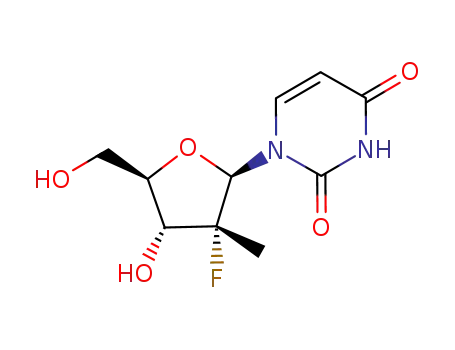
2'-deoxy-2'-fluoro-2'-methyluridine
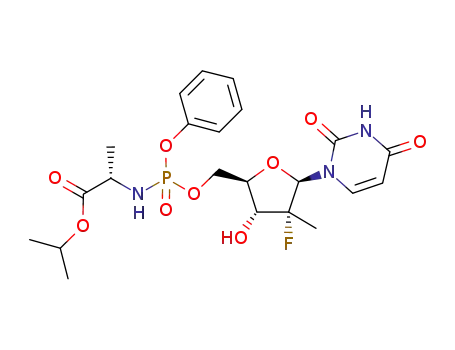
sofosbuvir
CAS:863329-66-2
CAS:143062-84-4
CAS:770-12-7