- Language:English
- English
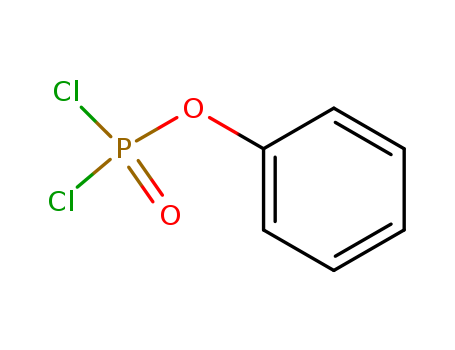

CasNo: 770-12-7
Molecular Formula: C6H5Cl2O2P
Appearance: colorless transparent liquid
|
Chemical Properties |
clear colourless to very slightly brown liquid |
|
Uses |
In organic synthesis for preparation of lactams, phosphate diesters and for oxidation reactions. |
|
General Description |
A liquid. May severely irritate skin, eyes and mucous membranes. |
|
Air & Water Reactions |
About the same density as water and moderately soluble in water. |
|
Reactivity Profile |
An hologenated organophosphate. Organophosphates are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides. |
|
Health Hazard |
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. |
|
Fire Hazard |
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. |
InChI:InChI=1/C6H5Cl2O2P/c7-11(8,9)10-6-4-2-1-3-5-6/h1-5H
Phenyl dichlorophosphate has been shown to be a highly efficient activating agent for dimethyl sulfoxide in the Pfitzner-Moffatt oxidation. Phenyl dichlorophosphate has been shown to be a highly efficient activating agent for dimethyl sulfoxide in the Pfitzner-Moffatt oxidation.
A series of Phenyl Phosphates (PPs) has ...
Herein, we wish to report that a convenient new procedure, making use of phenyl dichlorophosphate (3) as a key activating agent, has been developed in our laboratories to facilitate the title rearrangement at room temperature.
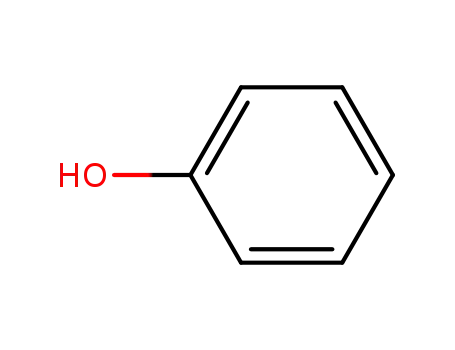
phenol

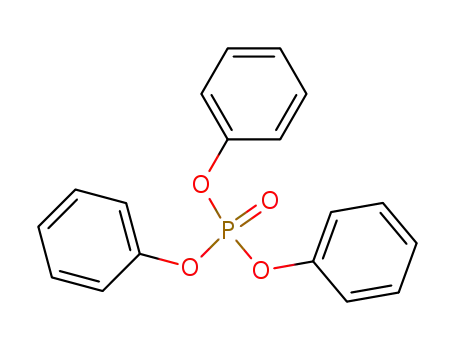
phosphoric acid triphenyl ester

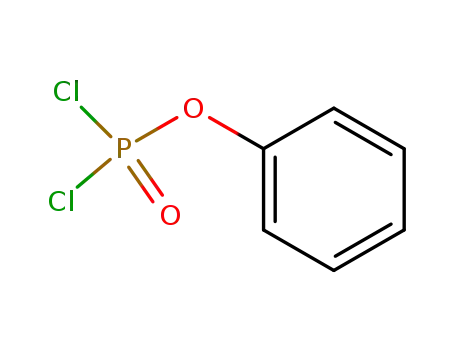
O-phenyl phosphorodichloridate

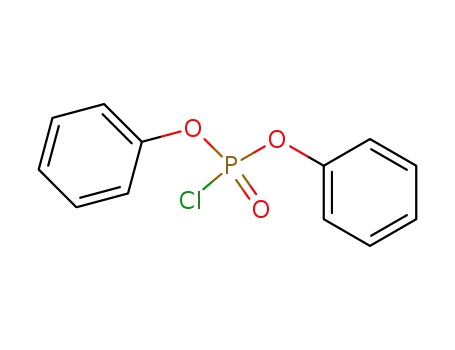
chlorophosphoric acid diphenyl ester
| Conditions | Yield |
|---|---|
|
With magnesium chloride; trichlorophosphate; In xylene; at 90 - 110 ℃; Kinetics; Product distribution; Rate constant;
|
|
|
With trichlorophosphate; at 102 - 240 ℃;
|
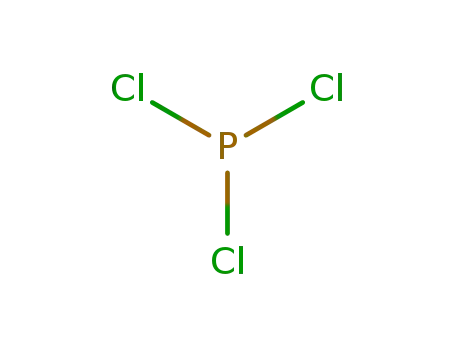
phosphorus trichloride


phenol


O-phenyl phosphorodichloridate


chlorophosphoric acid diphenyl ester
| Conditions | Yield |
|---|---|
|
at 140 ℃;
|

phenol
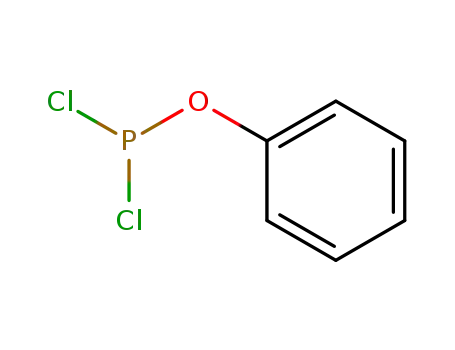
phenyl phosphorodichloridite
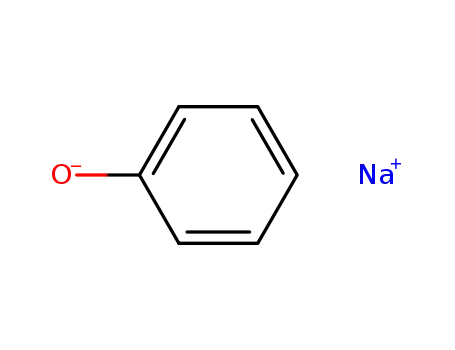
sodium phenoxide
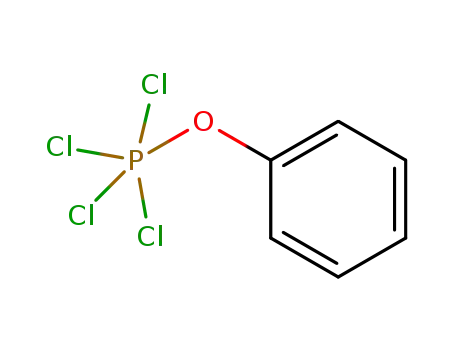
tetrachlorophenoxyphosphorane
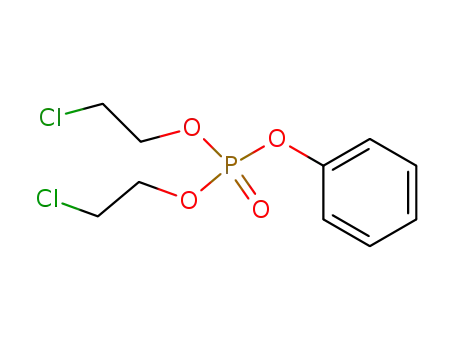
bis(2-chloroethyl) phenyl phosphate
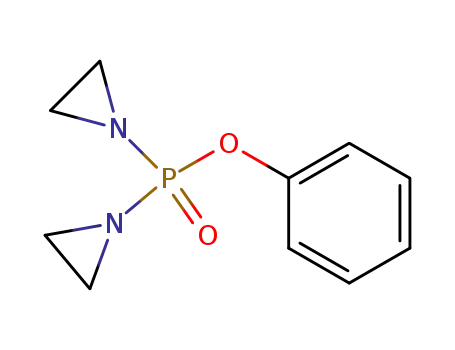
bis-aziridin-1-yl-phosphinic acid phenyl ester
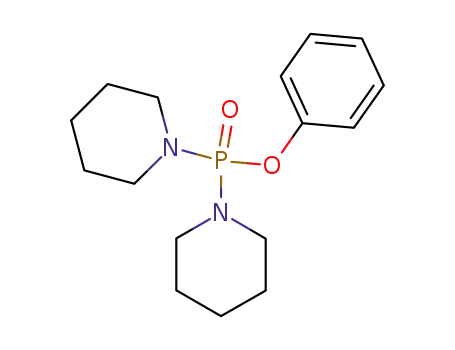
Phosphorsaeurephenylesterdipiperidid
di-morpholin-4-yl-phosphinic acid phenyl ester
CAS:151538-79-3
CAS:74124-79-1
CAS:863329-66-2