- Language:English
- English
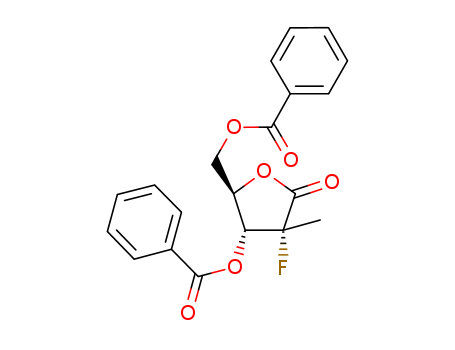

CasNo: 874638-80-9
Molecular Formula: C20H17FO6
|
Uses |
((2R,3R,4R)-3-(benzoyloxy)-4-fluoro-4-methyl-5-oxotetrahydrofuran-2-yl) methyl benzoate is used as neuromuscular blocking agent |
IUPAC Name: [(2R,3R,4R)-3-benzoyloxy-4-fluoro-4-methyl-5-oxooxolan-2-yl]methyl benzoate
Isomeric SMILES: C[C@]1([C@@H]([C@H](OC1=O)COC(=O)C2=CC=CC=C2)OC(=O)C3=CC=CC=C3)F
InChIKey: OUKYMZJNLWKCSO-JXXFODFXSA-N
InChI: InChI=1S/C20H17FO6/c1-20(21)16(27-18(23)14-10-6-3-7-11-14)15(26-19(20)24)12-25-17(22)13-8-4-2-5-9-13/h2-11,15-16H,12H2,1H3/t15-,16-,20-/m1/s1
The present disclosure is directed towar...
The invention discloses a preparation me...
The invention discloses a preparation me...
The invention provides a method for prep...
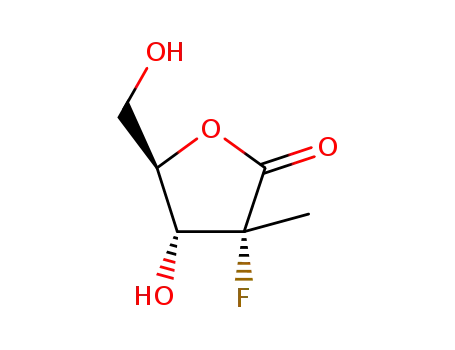
(3R,4R,5R)-3?fluoro?4?hydroxy?5?(hydroxymethyl)?3?methyltetrahydrofuran?2?one

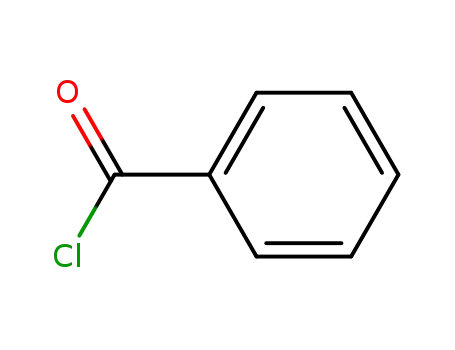
benzoyl chloride

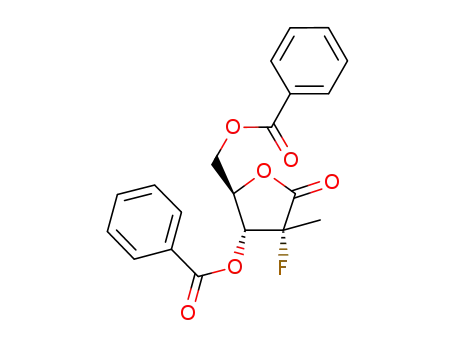
((2R,3R,4R)-3-benzoyloxy-4-fluoro-4-methyl-5-oxo-tetrahydrofuran-2-yl)methyl benzoate
| Conditions | Yield |
|---|---|
|
With pyridine; at 20 ℃; for 0.333333h;
|
87% |
|
With dmap; triethylamine; In dichloromethane; at 0 - 20 ℃; for 12h;
|
86% |
|
With dmap; triethylamine; In acetonitrile; at 5 - 20 ℃; for 2h; Temperature;
|
84.2% |
|
With dmap; triethylamine; In acetonitrile; at 20 - 25 ℃; for 4h; Inert atmosphere;
|
84% |
|
With dmap; triethylamine; In tetrahydrofuran; at 0 - 40 ℃; for 2h;
|
83.9% |
|
With dmap; triethylamine; In tetrahydrofuran; at 0 - 40 ℃; for 2h;
|
82.6% |
|
With dmap; triethylamine; In acetonitrile; at 10 - 40 ℃;
|
70.7% |
|
With pyridine; at 0 - 20 ℃; for 0.75h;
|
61.2% |
|
With pyridine; at 0 - 20 ℃; for 0.75h;
|
61.2% |
|
With pyridine; at 0 - 20 ℃; for 24h;
|
60% |
|
With dmap; triethylamine; In ethyl acetate; at -5 ℃; for 2h;
|
51.2% |
|
With dmap; triethylamine; In acetonitrile; at 10 - 30 ℃; for 5h; Large scale;
|
48.4% |
|
With pyridine; at 0 - 20 ℃; for 0.5h;
|
24 g |
|
With pyridine; at 20 ℃; for 0.5h; Solvent; Reagent/catalyst; Time; Concentration; Cooling with ice;
|
|
|
With pyridine; In acetonitrile; at 20 ℃; for 2h; Cooling with ice;
|
5.49 g |
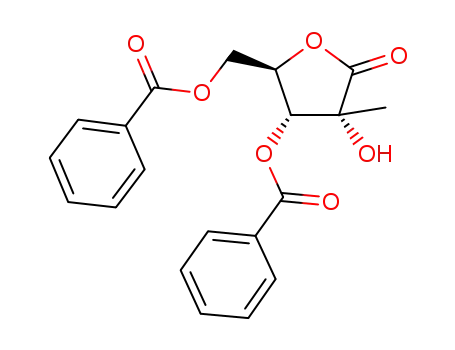
3,5-di-O-benzoyl-2-C-methyl-D-ribono-γ-lactone


((2R,3R,4R)-3-benzoyloxy-4-fluoro-4-methyl-5-oxo-tetrahydrofuran-2-yl)methyl benzoate
| Conditions | Yield |
|---|---|
|
3,5-di-O-benzoyl-2-C-methyl-D-ribono-γ-lactone; With pyridine; trifluoromethylsulfonic anhydride; In dichloromethane; at -40 - 20 ℃; for 1h;
With fluorosulfonyl fluoride; triethylamine tris(hydrogen fluoride); triethylamine; In acetonitrile; Cooling with ice;
|
78% |
|
Multi-step reaction with 3 steps
1.1: pyridine / dichloromethane / 0.08 h / -10 °C
1.2: 20 °C
2.1: dichloromethane; water; dimethyl sulfoxide / Cooling with ice
3.1: triethylamine; triethylamine tris(hydrogen fluoride); fluorosulfonyl fluoride / acetonitrile / Cooling with ice
With pyridine; fluorosulfonyl fluoride; triethylamine tris(hydrogen fluoride); triethylamine; In dichloromethane; water; dimethyl sulfoxide; acetonitrile;
|
|
|
Multi-step reaction with 4 steps
1.1: pyridine / dichloromethane / 0.08 h / -10 °C
1.2: 20 °C
2.1: water / 0.42 h
3.1: triethylamine / water; acetone / 45 °C
4.1: triethylamine; triethylamine tris(hydrogen fluoride); fluorosulfonyl fluoride / acetonitrile / Cooling with ice
With pyridine; fluorosulfonyl fluoride; triethylamine tris(hydrogen fluoride); triethylamine; In dichloromethane; water; acetone; acetonitrile;
|
|
|
Multi-step reaction with 2 steps
1: pyridine / dichloromethane / 1 h / -40 - 20 °C
2: triethylamine tris(hydrogen fluoride); triethylamine / acetonitrile / Cooling with ice
With pyridine; triethylamine tris(hydrogen fluoride); triethylamine; In dichloromethane; acetonitrile;
|
|
|
Multi-step reaction with 3 steps
1: triethylamine / dichloromethane / Cooling with ice
2: tetra-(n-butyl)ammonium iodide; potassium nitrite / dimethyl sulfoxide; water / 75 °C
3: triethylamine tris(hydrogen fluoride); triethylamine / acetonitrile / Cooling with ice
With potassium nitrite; tetra-(n-butyl)ammonium iodide; triethylamine tris(hydrogen fluoride); triethylamine; In dichloromethane; water; dimethyl sulfoxide; acetonitrile;
|
|
|
Multi-step reaction with 3 steps
1: pyridine / dichloromethane / 1 h / -40 - 20 °C
2: dimethyl sulfoxide / ethyl acetate
3: triethylamine tris(hydrogen fluoride); triethylamine / acetonitrile / Cooling with ice
With pyridine; dimethyl sulfoxide; triethylamine tris(hydrogen fluoride); triethylamine; In dichloromethane; ethyl acetate; acetonitrile;
|
|
|
Multi-step reaction with 4 steps
1: pyridine / dichloromethane / 1 h / -40 - 20 °C
2: 0.5 h / 20 °C
3: triethylamine / acetone / 50 °C
4: triethylamine tris(hydrogen fluoride); triethylamine / acetonitrile / Cooling with ice
With pyridine; triethylamine tris(hydrogen fluoride); triethylamine; In dichloromethane; acetone; acetonitrile;
|

(3R,4R,5R)-3?fluoro?4?hydroxy?5?(hydroxymethyl)?3?methyltetrahydrofuran?2?one

benzoyl chloride
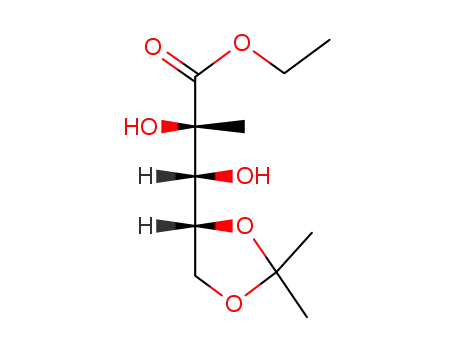
(2S,3R)-3-[(4R)-2,2-dimethyl-[1,3]dioxolane-4-yl]-2,3-dihydroxy-2-methylpropionic acid ethyl ester
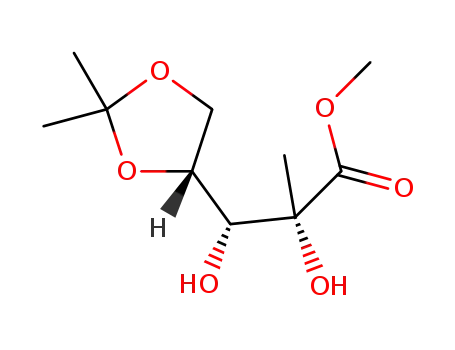
C10H18O6
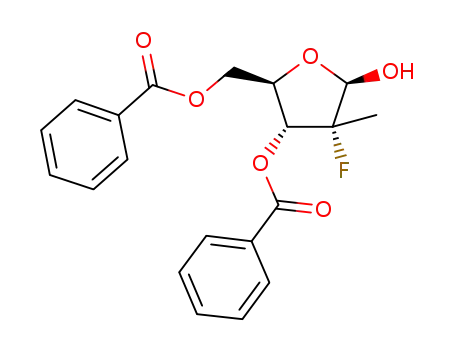
((2R,3R,4R,5R)-3-(benzoyloxy)-4-fluoro-5-hydroxy-4-methyltetrahydrofuran-2-yl)methyl benzoate
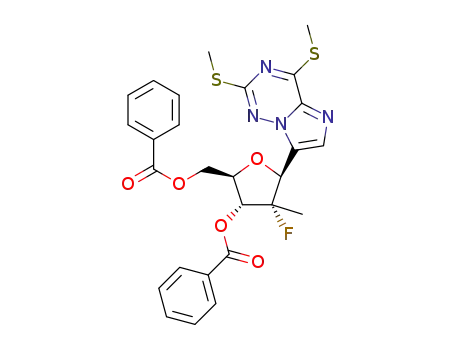
C27H25FN4O5S2
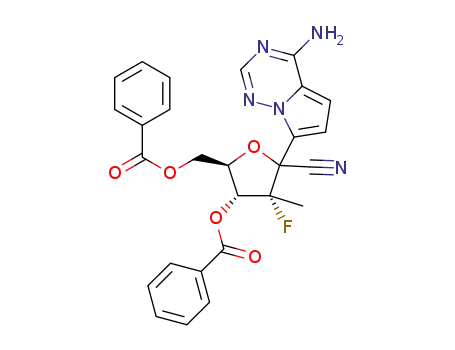
C27H22FN5O5
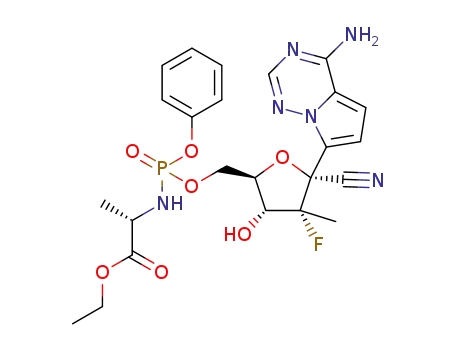
C24H28FN6O7P
CAS:99627-05-1
CAS:863329-66-2
CAS:62007-51-6