- Language:English
- English


CasNo: 22364-68-7
Molecular Formula: C9H9N
Appearance: clear colorless to orange-reddish liquid
|
Chemical Properties |
clear colorless to orange-reddish liquid |
|
Uses |
o-Tolylacetonitrile is used as an important raw material and intermediate in organic Synthesis, pharmaceuticals, agrochemicals and dyestuff. It is also used as an organic and chemical intermediate. |
|
Definition |
ChEBI: A nitrile that is acetonitrile where one of the methyl hydrogens is substituted by a 2-methylphenyl group. |
|
Synthesis Reference(s) |
Synthesis, p. 40, 1987 DOI: 10.1055/s-1987-27834 |
InChI:InChI=1/C7H12Cl2N2O2/c8-4-6(12)10-2-1-3-11-7(13)5-9/h1-5H2,(H,10,12)(H,11,13)
The photooxygenation of 1,4-cyclohexadie...
Cyano-containing compounds constitute im...
The invention belongs to the technical f...
A titanium-catalyzed mono-decyanation of...
C22H18N2O4


2-tolylmethylnitrile

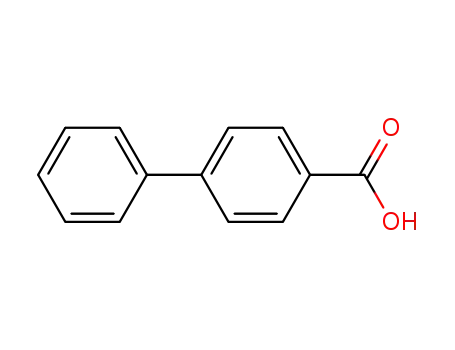
biphenyl-4-carboxylic acid
| Conditions | Yield |
|---|---|
|
With
2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride;
In
benzene;
Heating;
|
96% 94% |
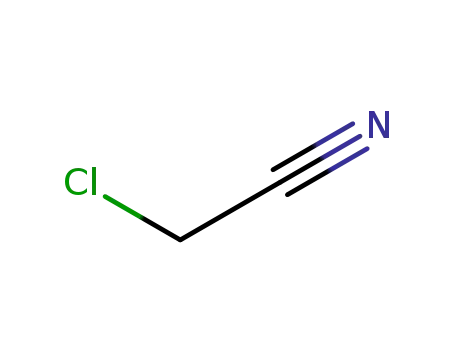
chloroacetonitrile

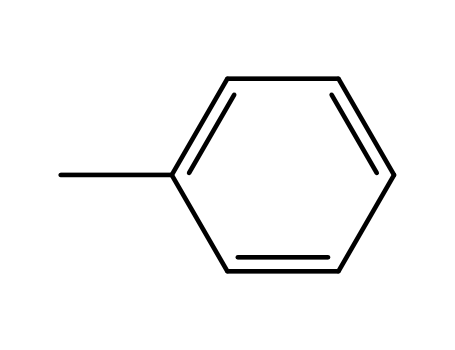
toluene

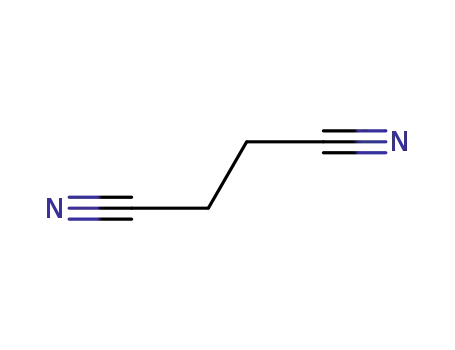
butanedinitrile


3-methylbenzyl cyanide


(4-methylphenyl)acetonitrile


1,1'-(1,2-ethanediyl)bisbenzene

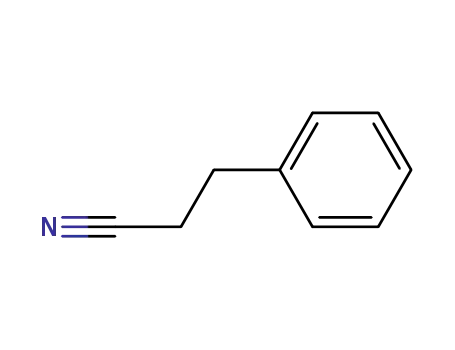
dihydrocinnamonitrile


2-tolylmethylnitrile
| Conditions | Yield |
|---|---|
|
In
acetonitrile;
Product distribution;
Mechanism;
Irradiation;
different concentration ratios;
|
0.7% 6.4% 2.1% |
|
In
acetonitrile;
for 22h;
Kinetics;
Product distribution;
Mechanism;
Irradiation;
|
6.4% 2.1% 0.7% |
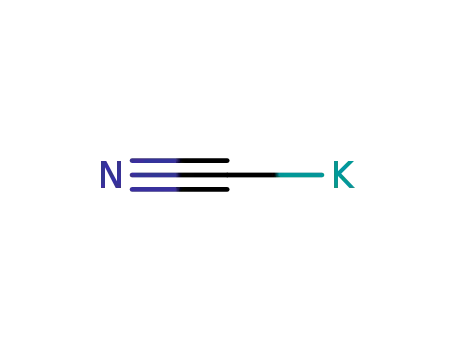
potassium cyanide
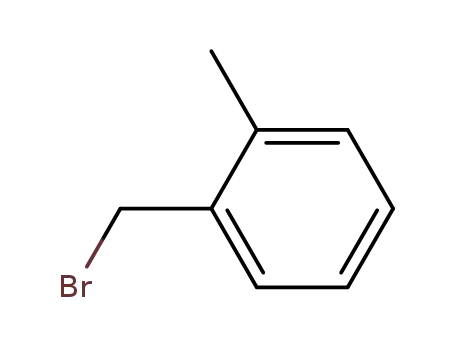
2-methylbenzyl bromide
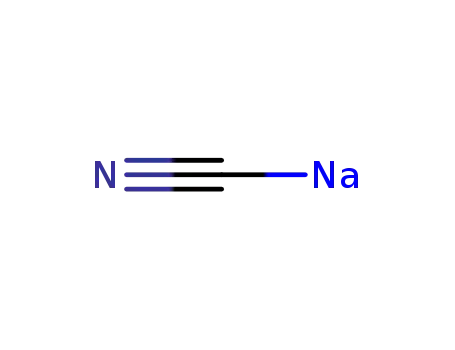
sodium cyanide
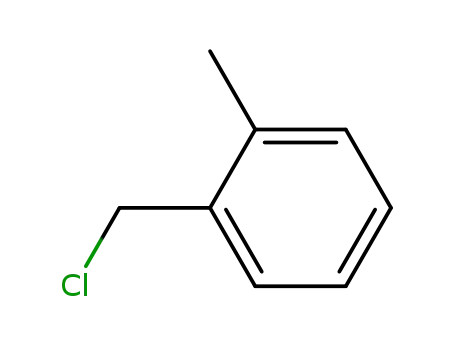
1-chloromethyl-2-methylbenzene
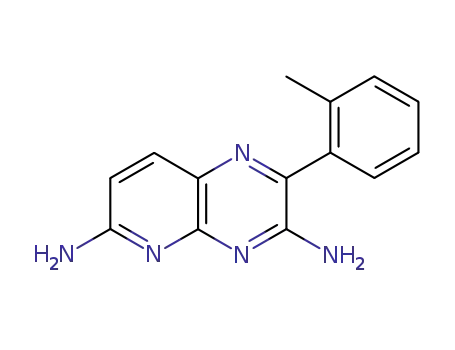
2-o-tolyl-pyrido[2,3-b]pyrazine-3,6-diyldiamine
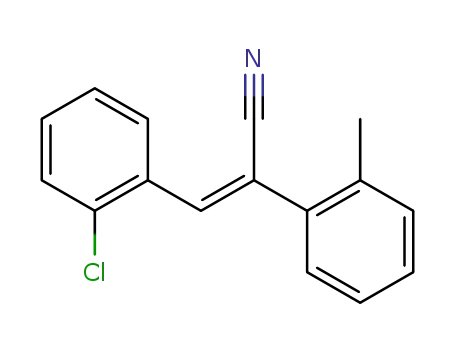
3c-(2-chloro-phenyl)-2-o-tolyl-acrylonitrile
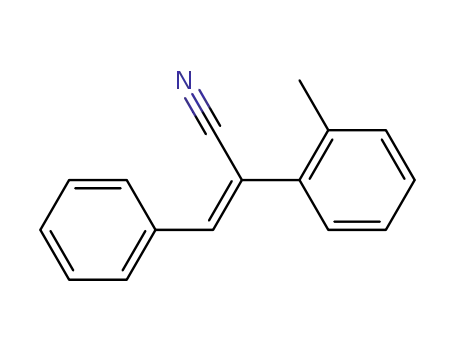
α-cyano-2-methylstilbene
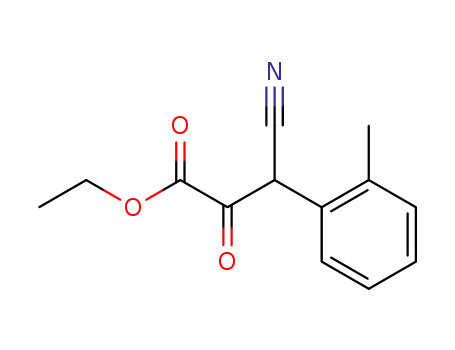
3-cyano-2-oxo-3-o-tolyl-propionic acid ethyl ester
CAS:138071-82-6
CAS:38083-17-9
CAS:104-77-8
CAS:934816-82-7