- Language:English
- English
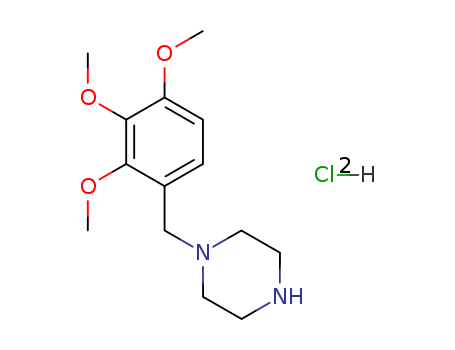

CasNo: 13171-25-0
Molecular Formula: C14H24Cl2N2O3
Appearance: crystalline solid
|
Description |
Trimetazidine dihydrochloride is the dihydrochloride salt of Trimetazidine. It is a drug used for the treatment of angina pectoris. It is capable of improving the left ventricular function in diabetic patients suffering coronary heart disease as well as treating the symptoms of patients suffering heart failure of different etiologies. It takes effects through acting as an anti- ischemic metabolic agent being capable of improving the utilization efficiency of myocardial glucose by inhibiting the metabolism of fatty acid (through inhibiting the activity of mitochondrial long- chain 3-ketoacyl coenzyme A Thiolase). The shift from fatty acid oxidation to glucose oxidation optimizes cellular energy process in cells exposed to hypoxia or ischaemia, further maintaining the intracellular ATP levels and ensuring the normal function of ionic pumps and transmembrane sodium-potassium flow to maintain cellular homeostasis. |
|
References |
Fragasso, G, et al. "A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. " Journal of the American College of Cardiology 48.5 (2006):992. Tuunanen, H, et al. "Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy."Circulation 118.12(2008):1250. Kantor, P. F., et al. "The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase."Circulation Research 86.5(2000):580. Stanley, W. C., and M. Marzilli. "Metabolic therapy in the treatment of ischaemic heart disease: the pharmacology of trimetazidine. "Fundamental & Clinical Pharmacology 17.2(2003):133–145. Kantor, Paul F., et al. "The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase." Circulation research 86.5 (2000): 580-588. |
|
Chemical Properties |
Crystalline Solid |
|
Uses |
Antianoxic |
InChI:InChI=1/C14H22N2O3/c1-17-12-5-4-11(13(18-2)14(12)19-3)10-16-8-6-15-7-9-16/h4-5,15H,6-10H2,1-3H3/p+2
The invention provides a synthesis metho...
The invention discloses a method for pre...
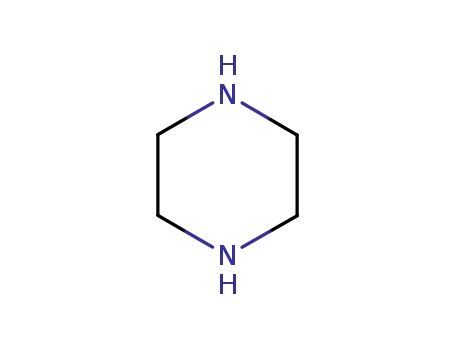
piperazine

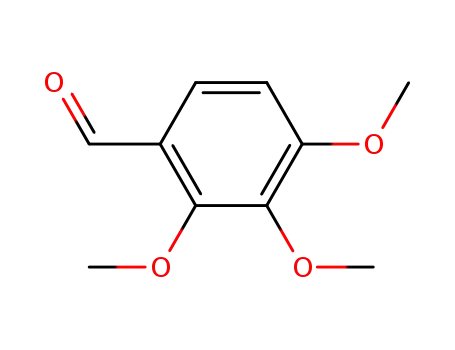
2,3,4-trimethoxybenzaldehyde

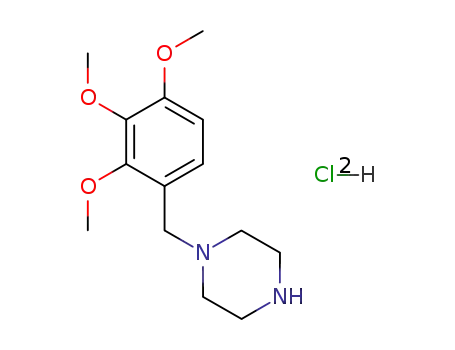
trimetazidine dihydrochloride
| Conditions | Yield |
|---|---|
|
piperazine;
With
acetic acid;
In
methanol;
at 40 ℃;
for 0.5h;
2,3,4-trimethoxybenzaldehyde;
With
hydrogen; cetyltrimethylammonim bromide; nickel;
In
methanol;
at 35 - 60 ℃;
for 4h;
under 3750.38 - 6000.6 Torr;
Sealed tube;
With
hydrogenchloride;
In
water;
for 0.5h;
pH=2;
Concentration;
|
95% |
|
piperazine; 2,3,4-trimethoxybenzaldehyde;
With
Lindlar Pd catalyst; hydrogen;
In
ethanol;
at 60 ℃;
for 16h;
under 15001.5 Torr;
Large scale;
With
hydrogenchloride;
In
acetone;
at 10 ℃;
for 2h;
Reagent/catalyst;
Solvent;
Temperature;
Large scale;
|
92.5% |
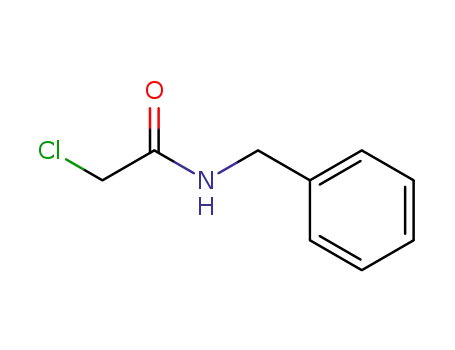
N-benzyl-2-chloroacetamide


trimetazidine dihydrochloride

![N-Benzyl-2-[4-(2,3,4-trimethoxy-benzyl)-piperazin-1-yl]-acetamide](/upload/2023/6/d3c9da19-1017-46b0-a463-05bb7750b1a5.png)
N-Benzyl-2-[4-(2,3,4-trimethoxy-benzyl)-piperazin-1-yl]-acetamide
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
In
acetonitrile;
at 70 ℃;
|
76% |

piperazine

2,3,4-trimethoxybenzaldehyde
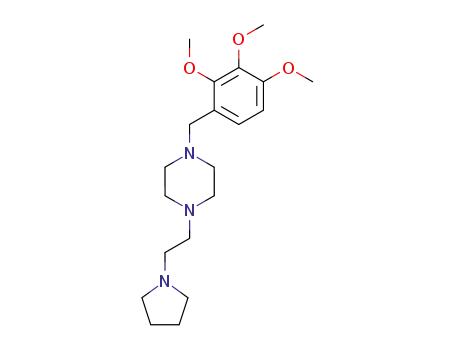
1-<2-(pyrrolidino)ethyl>-4-(2,3,4-trimethoxybenzyl)piperazine
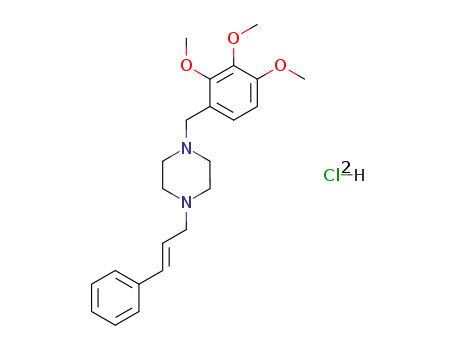
1-((E)-3-Phenyl-allyl)-4-(2,3,4-trimethoxy-benzyl)-piperazine; hydrochloride

1-(pyrrolidinocarbonylmethyl)-4-(2,3,4-trimethoxybenzyl)piperazine
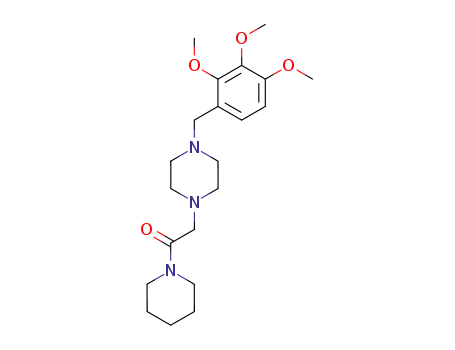
1-Piperidin-1-yl-2-[4-(2,3,4-trimethoxy-benzyl)-piperazin-1-yl]-ethanone
CAS:38083-17-9
CAS:120011-70-3
CAS:109384-19-2
CAS:104-77-8