- Language:English
- English
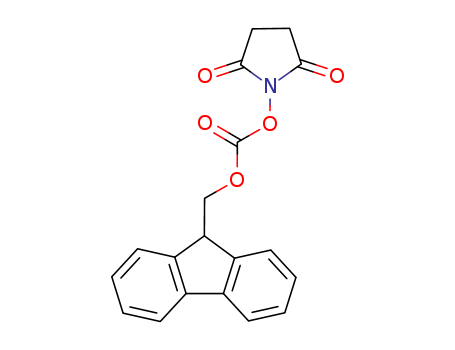

CasNo: 82911-69-1
Molecular Formula: C19H15NO5
Appearance: White powder
|
Chemical Properties |
White powder |
|
Uses |
N-(9-Fluorenylmethoxycarbonyloxy)succinimide is used in conjunction with Isothiocyanate to conduct partial Edman Degradation on biologically active peptides. |
|
Purification Methods |
Recrystallise the carbonate from CHCl3/Et2O, or from pet ether (b 40-60o). [Pauet Can J Chem 60 976 1982, Lapatsaris et al. Synthesis 671 1983.] |
InChI:InChI=1/C19H15NO5/c21-17-9-10-18(22)20(17)25-19(23)24-11-16-14-7-3-1-5-12(14)13-6-2-4-8-15(13)16/h1-8,16H,9-11H2
The invention relates to the technical f...
Jadomycin Oct (1) was isolated from Stre...
Bz(NO2)-Orn(Boc)-OCH2CN was synthesized ...
The applicability of terminated oligomer...
1-hydroxy-pyrrolidine-2,5-dione

(fluorenylmethoxy)carbonyl chloride

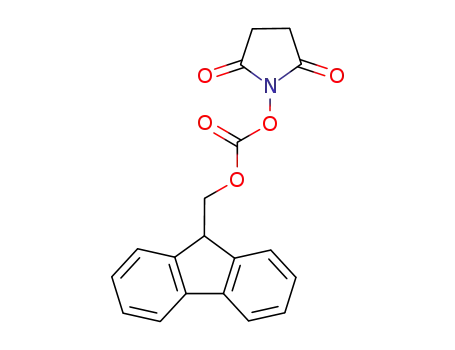
N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
1,4-dioxane;
for 1h;
Ambient temperature;
|
96% |
|
With
sodium carbonate;
In
water; toluene;
at 25 - 30 ℃;
for 2h;
Reagent/catalyst;
Solvent;
|
56.3% |
|
With
sodium carbonate;
In
water; acetone;
for 0.5h;
|
|
|
With
sodium carbonate;
In
water; acetone;
at -10 ℃;
for 1.5h;
|
|
|
With
triethylamine;
In
1,4-dioxane;
|
|
|
With
diisopropylamine; dicyclohexyl-carbodiimide;
In
dichloromethane;
at 0 - 20 ℃;
for 16h;
Inert atmosphere;
|
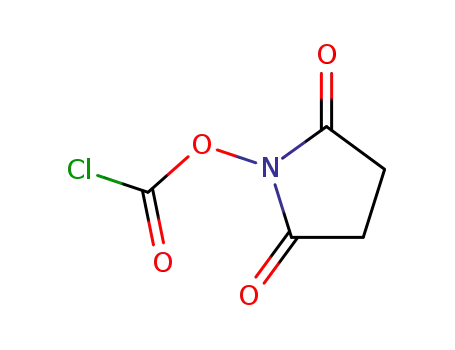
2,5-dioxopyrrolidin-1-yl carbonochloridate

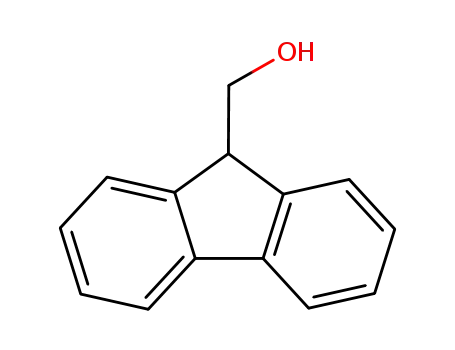
9-Fluorenylmethanol


N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide
| Conditions | Yield |
|---|---|
|
With
pyridine;
In
dichloromethane;
for 5h;
Ambient temperature;
|
72% |

1-hydroxy-pyrrolidine-2,5-dione

(fluorenylmethoxy)carbonyl chloride

2,5-dioxopyrrolidin-1-yl carbonochloridate

9-Fluorenylmethanol
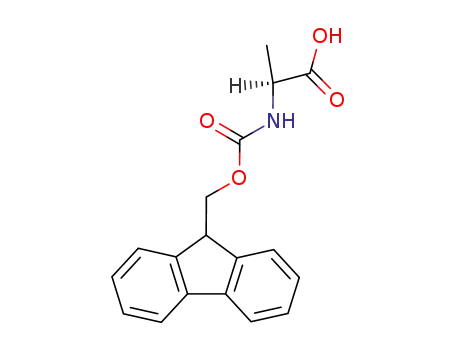
N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-alanine
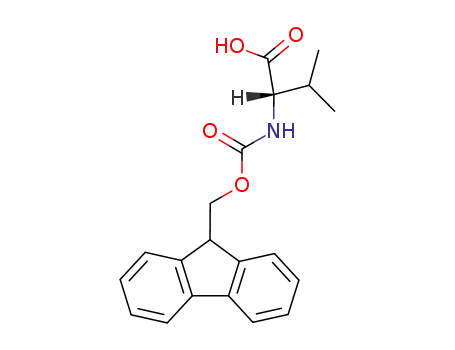
Fmoc-Val-OH
N-(9H-fluoren-9-ylmethoxycarbonyl)-L-serine

Fmoc-Thr-OH
CAS:138071-82-6
CAS:38083-17-9
CAS:538-75-0
CAS:6038-19-3