- Language:English
- English
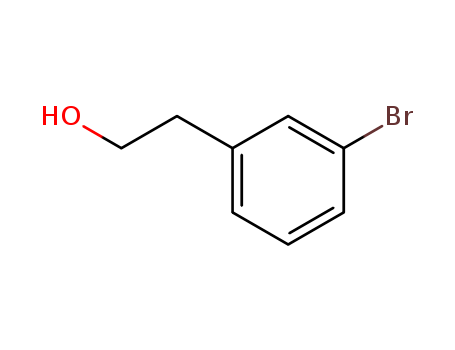

CasNo: 28229-69-8
Molecular Formula: C8H9BrO
|
Chemical Properties |
clear colorless liquid |
|
Uses |
m-Bromobenzeneethanol may be used as a starting material in the synthesis of its mesylate derivative (m-Br-C6H4CH2CH2OMs) by treatment with mesyl chloride. m-Bromobenzeneethanol finds applications in various fields, including organic synthesis and pharmaceutical research. It can serve as a starting material or intermediate for the synthesis of other organic compounds. The presence of the bromine atom makes it useful for reactions involving nucleophilic substitution or coupling reactions. Additionally, the alcohol group provides potential for further chemical modifications or functionalization. |
|
General Description |
m-Bromobenzeneethanol, also known as 3-bromo-phenethyl alcohol, i is a phenethyl alcohol derivative. Its enthalpy of vaporization at boiling point has been determined. |
IUPAC Name: 2-(3-bromophenyl)ethanol
Isomeric SMILES: C1=CC(=CC(=C1)Br)CCO
InChIKey: PTTFLKHCSZSFOL-UHFFFAOYSA-N
InChI: InChI=1S/C8H9BrO/c9-8-3-1-2-7(6-8)4-5-10/h1-3,6,10H,4-5H2
This work reports the synthesis, radiola...
Cheap and readily available aqueous form...
A series of CAPE derivatives with mono-s...
The hydroxy group of commercial 3-bromophenethyl alcohol was protected by reaction with tert-butyl(chloro)dimethylsilane (TBDMS-Cl) and treated with trimethyl borate and t-BuLi to …
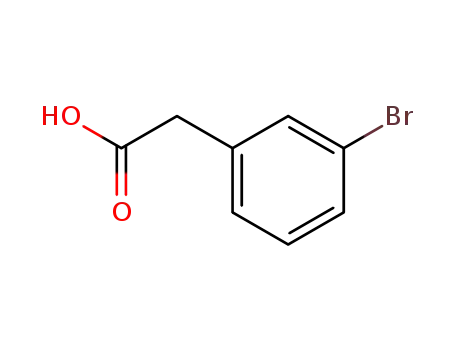
3-Bromophenylacetic acid

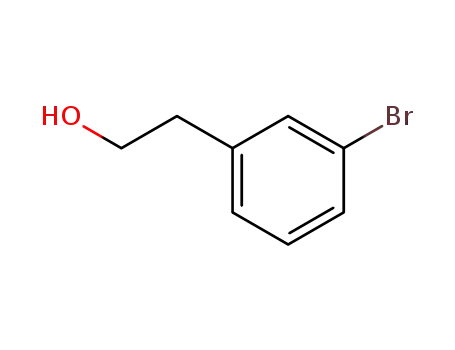
2-(3-bromophenyl)ethan-1-ol
| Conditions | Yield |
|---|---|
|
With borane-THF; In tetrahydrofuran; at 0 ℃; for 4h;
|
93% |
|
With borohydride-tetrahydrofuran complex; at 20 ℃; for 2h;
|
80% |
|
3-Bromophenylacetic acid; With dimethylsulfide borane complex; In tetrahydrofuran; at 0 - 20 ℃; for 20h;
With water; In tetrahydrofuran;
|
79% |
|
With borane; In tetrahydrofuran; 1.) 0 deg C, 30 min, 2.) 25 deg C, 2 h;
|
|
|
With dimethylsulfide borane complex;
|
|
|
With diborane; In tetrahydrofuran; at 20 ℃; for 16h;
|
9.0 g |
|
3-Bromophenylacetic acid; With lithium aluminium tetrahydride; In tetrahydrofuran; at 0 ℃; for 2h; Heating / reflux;
With water; Rochelle's salt; In tetrahydrofuran;
|
|
|
With benzophenone; potassium; In tetrahydrofuran; methanol;
|
|
|
In tetrahydrofuran; (2S)-N-methyl-1-phenylpropan-2-amine hydrate;
|
|
|
3-Bromophenylacetic acid; With lithium aluminium tetrahydride; In tetrahydrofuran; at 0 ℃; for 2h; Heating / reflux;
With Rochelle's salt; In tetrahydrofuran; water; for 0.5h;
|
|
|
With lithium aluminium tetrahydride; In diethyl ether; Reflux;
|
|
|
3-Bromophenylacetic acid; With lithium aluminium tetrahydride; In tetrahydrofuran; at 0 - 20 ℃; for 2h;
With water; In tetrahydrofuran;
|
|
|
With dimethylsulfide borane complex; In tetrahydrofuran; for 1h; Inert atmosphere; Reflux;
|
|
|
With borane-THF; In tetrahydrofuran; at 20 ℃;
|
|
|
With lithium aluminium tetrahydride; In tetrahydrofuran; at 0 - 20 ℃;
|
|
|
With lithium aluminium tetrahydride; In tetrahydrofuran; at 0 ℃; for 4.5h; Reflux;
|
|
|
With diborane; In tetrahydrofuran;
|
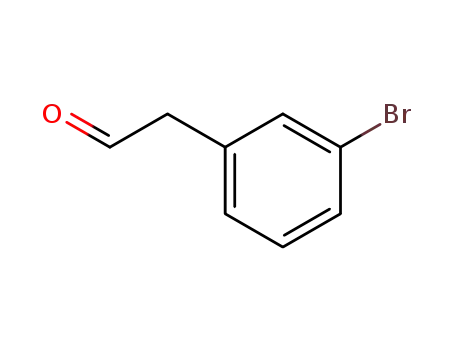
2-(3-bromophenyl)acetaldehyde


2-(3-bromophenyl)ethan-1-ol
| Conditions | Yield |
|---|---|
|
With sodium tetrahydroborate; In ethanol; for 1h; Inert atmosphere;
|
66% |
|
With glycerol; In dimethyl sulfoxide; at 0 ℃; for 0.5h; Inert atmosphere;
|
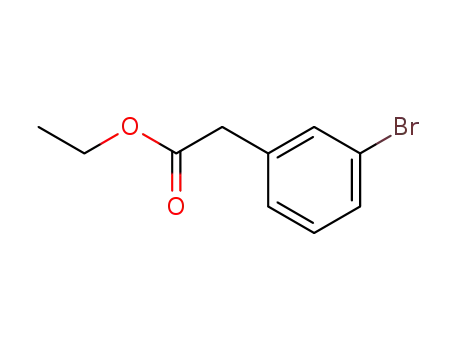
ethyl 2-(3-bromophenyl)acetate

3-Bromophenylacetic acid
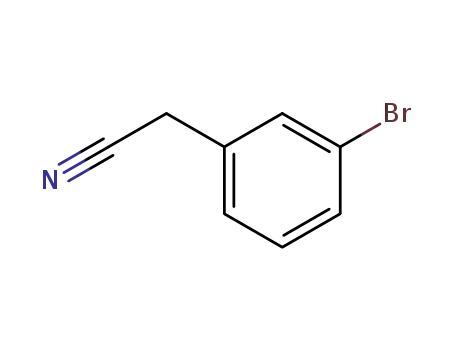
(3-bromophenyl)acetonitrile
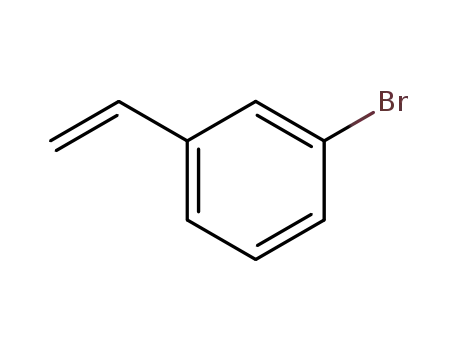
3-bromostyrene

3-bromostyrene
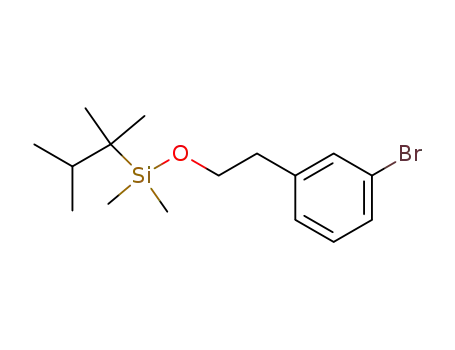
<2-(3-bromophenyl)ethoxy>dimethyl(1,1,2-trimethylpropyl)silane
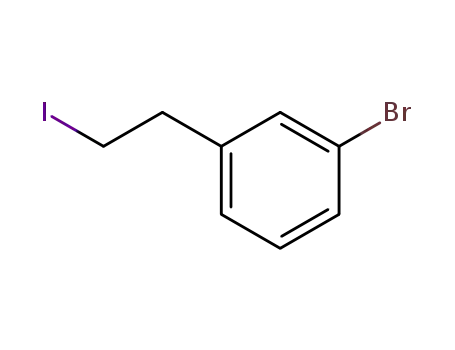
1-bromo-3-(2'-iodoethyl)benzene
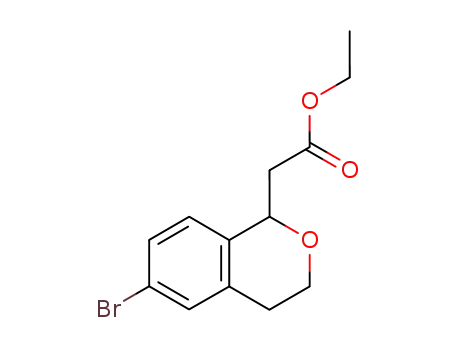
ethyl (6-bromoisochroman-1-yl)acetate
CAS:1953-04-4
CAS:20826-04-4
CAS:1074-16-4
CAS:30595-79-0