- Language:English
- English
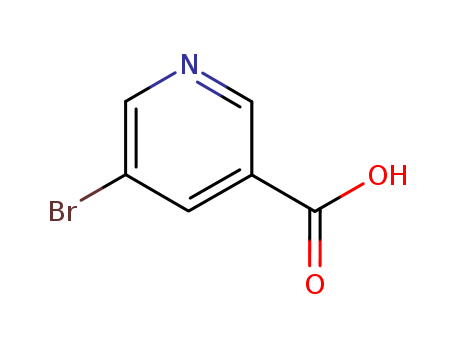

CasNo: 20826-04-4
Molecular Formula: C6H4BrNO2
Appearance: Light yellow cryst.
|
Chemical Properties |
Light yellow Cryst |
|
Uses |
5-Bromopyridine-3-carboxylic acid (5-bromonicotinic acid) was used in the synthesis of 3-guanidinomethyl-5-iodopyridine. |
|
Purification Methods |
The acid is recrystallised from H2O and then from EtOH using charcoal. The amide has m 219-219.5o (from aqueous EtOH), and the methyl ester, prepared by addition of ethereal diazomethane, can be purified by sublimation in a vacuum and has m 98-99o. The acid chloride also can be sublimed in vacuo and has m 74-75o and gives the methyl ester in MeOH. [Graf J Prakt Chem 138 244 1933, Bachman & Micucci J Am Chem Soc 70 2381 1948, Garcia et al. J Am Chem Soc 82 4430 1960, Misic-Vokovic et al. J Chem Soc 34 1978, Beilstein 22/2 V 181.] |
IUPAC Name: 5-bromopyridine-3-carboxylic acid
Isomeric SMILES: C1=C(C=NC=C1Br)C(=O)O
InChIKey: FQIUCPGDKPXSLL-UHFFFAOYSA-N
InChI: InChI=1S/C6H4BrNO2/c7-5-1-4(6(9)10)2-8-3-5/h1-3H,(H,9,10)
We report the formation of four novel multifunctional coordination compounds based on 5-bromonicotinic acid and different lanthanide(III) ions (Dy, Tb, Yb and Nd), synthesized by simple hydrothermal routes.
Two types of monoclonal antibodies were ...
Disclosed herein are inhibitors of gene ...
Two new metal–organic frameworks based on 5-bromonicotinic acid complexes [Cd(5-BrNic)2]n (1) and [Co(5-BrNic)2(H2O)]n (2) have been synthesized by hydrothermal reactions of this ligand with cadmium and cobalt metallic(II) salts in the presence of water.
5-bromo-3-pyridine carboxylic acid methyl ester

5-bromo-3-pyridinecarboxylic acid
| Conditions | Yield |
|---|---|
|
With potassium hydroxide; In methanol; water; for 12h; Ambient temperature;
|
91% |
|
With water; sodium hydroxide; In tetrahydrofuran; at 20 ℃; for 0.166667h;
|
33% |
3-pyridinecarbonyl chloride


5-bromo-3-pyridinecarboxylic acid
| Conditions | Yield |
|---|---|
|
With bromine; at 0 - 155 ℃; for 10h;
|
94% |
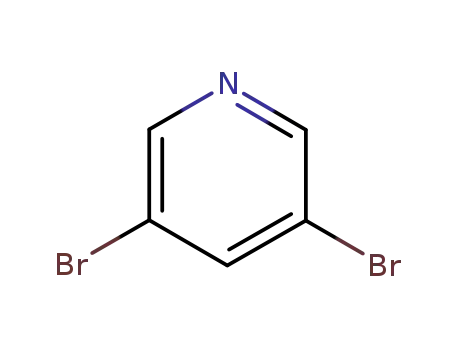
3,5-dibromopyridine
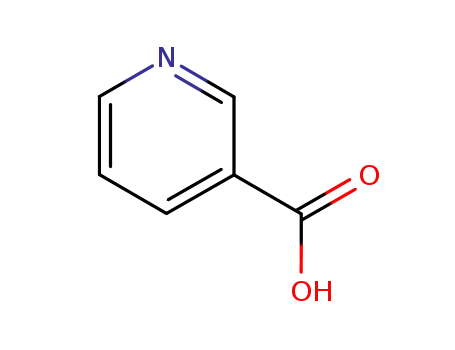
nicotinic acid
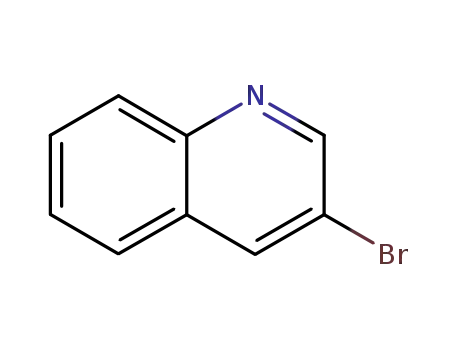
3-bromoquinoline
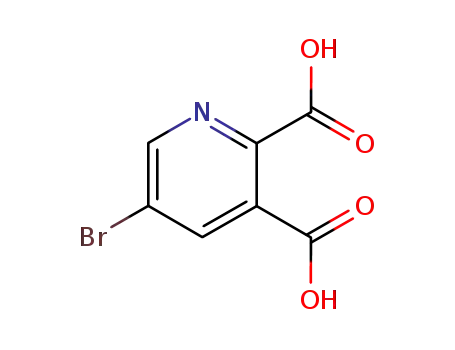
5-Bromo-pyridine-2,3-dicarboxylic acid
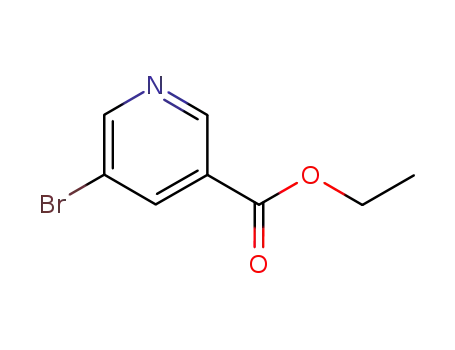
ethyl 5-bromo-3-pyridinecarboxylate
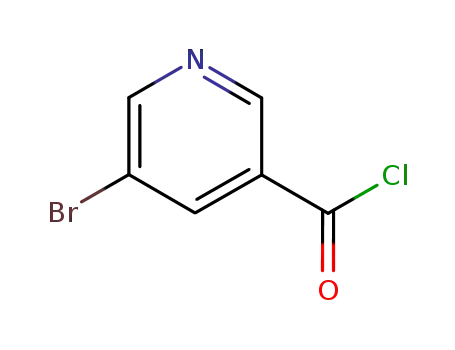
5-bromonicotinoyl chloride
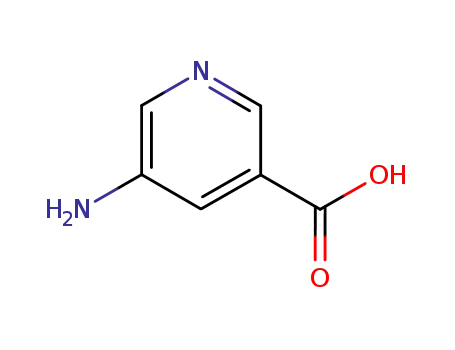
5-aminonicotinic acid
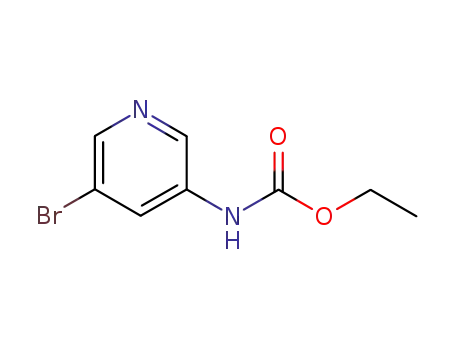
3-bromo-5-(ethoxycarbonyl)aminopyridine
CAS:1953-04-4
CAS:146939-27-7
CAS:144171-61-9