- Language:English
- English
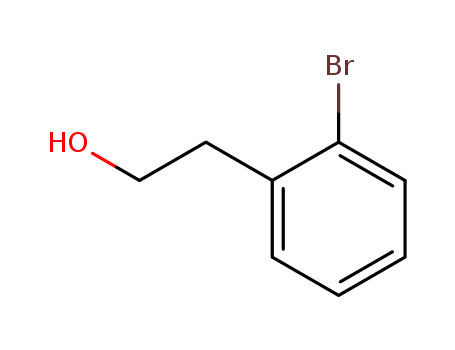

CasNo: 1074-16-4
Molecular Formula: C8H9BrO
Appearance: clear slightly yellow liquid
2-(2-Bromophenyl)Ethanol is a useful chemical compound. It is also known as α-(2-bromophenyl)ethanol.
|
Chemical Properties |
clear slightly yellow liquid |
|
Uses |
2-Bromophenethylalcohol is commonly used as a building block in the synthesis of various organic compounds, including pharmaceuticals and agrochemicals. It is also used as a chiral auxiliary in asymmetric synthesis. The compound has a chiral center and can exist in two enantiomeric forms, making it useful in the production of enantiopure compounds. 2-(2-Bromophenyl)Ethanol has a wide range of applications in the chemical industry and is an important intermediate in organic synthesis. |
|
General Description |
2-Bromophenethyl alcohol is a phenethyl alcohol derivative. It participates in the preparation of novel P-chirogenic phosphines with a sulfur-chelating arm (P*,S-hybrid ligand). |
IUPAC Name: 2-(2-bromophenyl)ethanol
Isomeric SMILES: C1=CC=C(C(=C1)CCO)Br
InChIKey: ADLOWZRDUHSVRU-UHFFFAOYSA-N
InChI: InChI=1S/C8H9BrO/c9-8-4-2-1-3-7(8)5-6-10/h1-4,10H,5-6H2
The binary hydride, diisobutylaluminum b...
SUMOylation is a reversible post-transla...
The treatment of 2-(2-vinylphenyl)acetal...
The synthesized compounds were then evaluated for their antifungal activity against several strains of fungi, including Candida albicans and Aspergillus fumigatus, which are common causes of fungal infections in humans. The study suggests that 2-(2-bromophenyl)ethanol derivatives have potential as a starting point for the development of novel antifungal agents.
2-phenylethanol

4-bromophenethanol

2-bromophenylethyl alcohol
| Conditions | Yield |
|---|---|
|
With pyridinium hydrobromide perbromide; In water; at 20 ℃; Title compound not separated from byproducts;
|
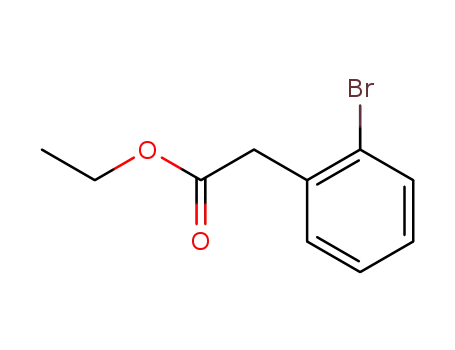
ethyl 2-(2-bromophenyl)ethanoate


2-bromophenylethyl alcohol
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water;
|
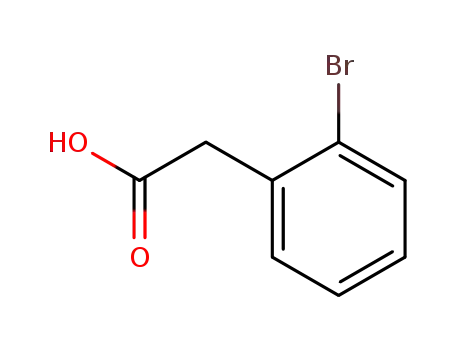
ortho-bromophenylacetic acid
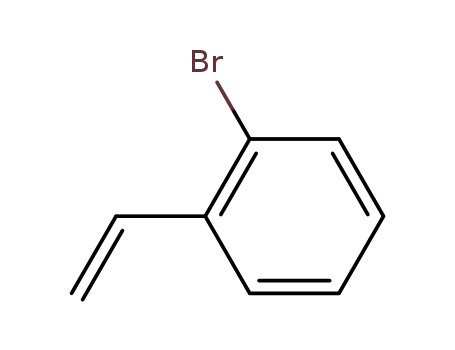
2-bromostyrene

2-phenylethanol
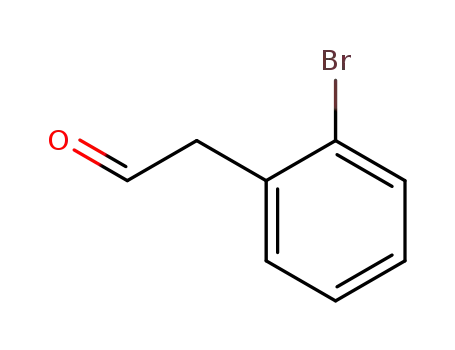
2-bromophenylacetaldehyde
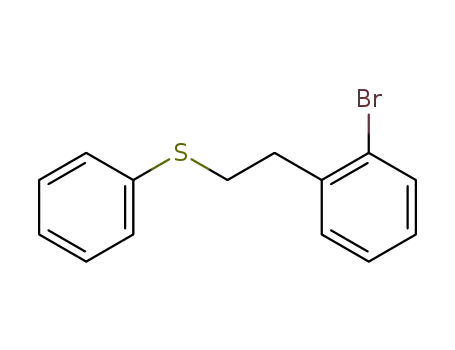
2-<2-Phenylmercapto-ethyl>-brombenzol
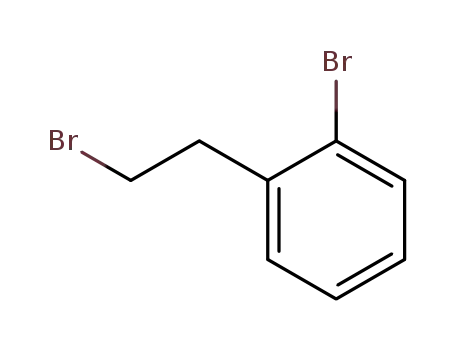
1-bromo-2-(2-bromoethyl)benzene
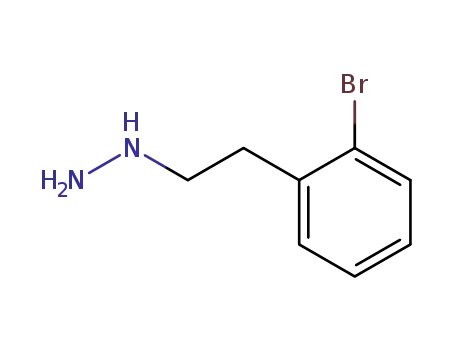
o-Brom-β-phenethylhydrazin
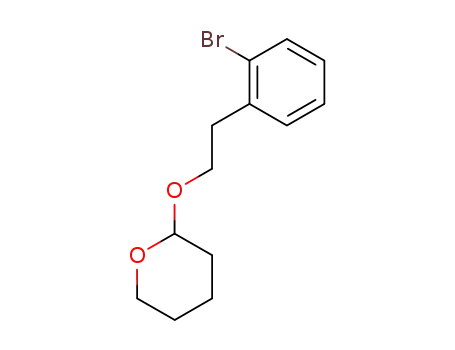
2-(2-bromophenyl)ethyl tetrahydro-2H-pyran-2-yl ether
CAS:1953-04-4
CAS:20826-04-4
CAS:156-41-2
CAS:28229-69-8