- Language:English
- English
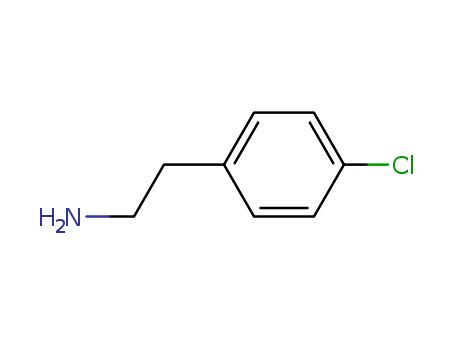

CasNo: 156-41-2
Molecular Formula: C8H10ClN
Appearance: clear almost colorless liquid.
|
Chemical Properties |
Dark Solid |
|
Uses |
4-chlorophenethylamine has been used as a reactant in the preparation of isoalloxazine derivatives as cholinesterase inhibitors with a potential use in Alzheimer therapy. 4-chlorophenethylamine is commercially available. |
IUPAC Name: 2-(4-chlorophenyl)ethanamine
Isomeric SMILES: C1=CC(=CC=C1CCN)Cl
InChIKey: SRXFXCKTIGELTI-UHFFFAOYSA-N
InChI: InChI=1S/C8H10ClN/c9-8-3-1-7(2-4-8)5-6-10/h1-4H,5-6,10H2
Compounds of the 4-chlorophenethyl amine part of carpropamid were replaced with alkyl, cycloalkyl and substituted phenylalkyl amines. The amines were obtained from the corresponding ketoximes using a reduction system of sodium borohydride with molybdenum oxide as the additive.
The conversion of carboxylic acids, such...
Continuous-flow synthesis of baclofen pr...
The α-methyl analogues of dopamine, norepinephrine, phenethylamine, tyramine, or 4-chlorophenethylamine also inhibited kynuramine oxidation.
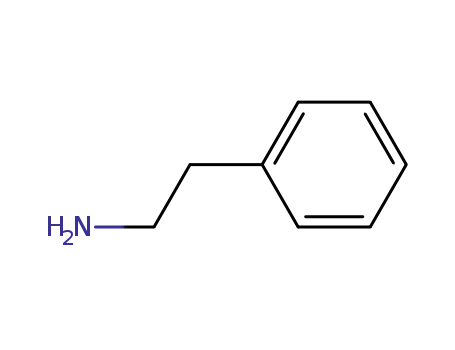
phenethylamine

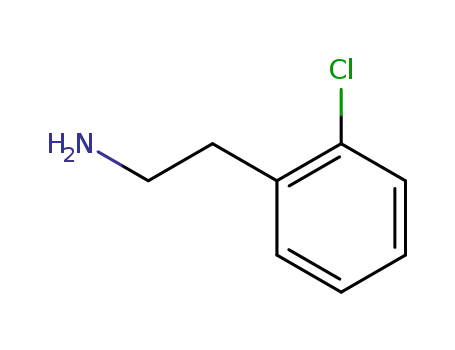
2-(2-chlorophenyl)ethanamine

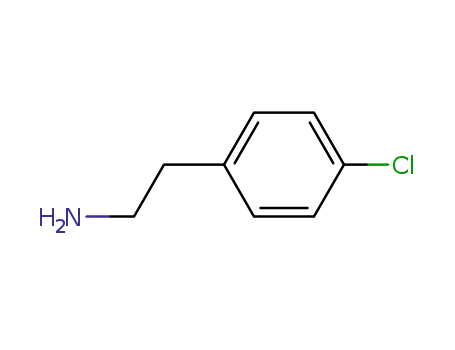
4-chlorophenylethylamine
| Conditions | Yield |
|---|---|
|
With chlorine; In tetrachloromethane; at 25 ℃; Further Variations:; Solvents; Product distribution;
|
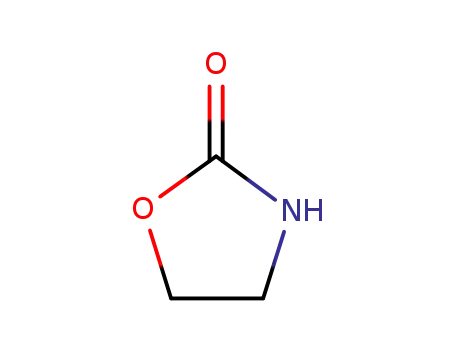
dimethylenecyclourethane

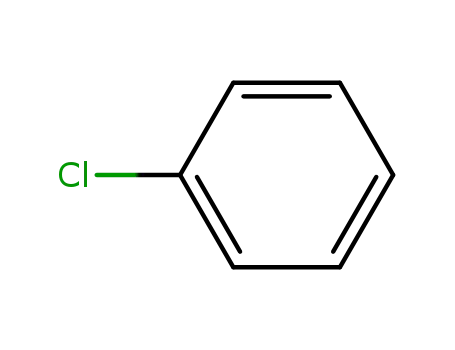
chlorobenzene


2-(2-chlorophenyl)ethanamine

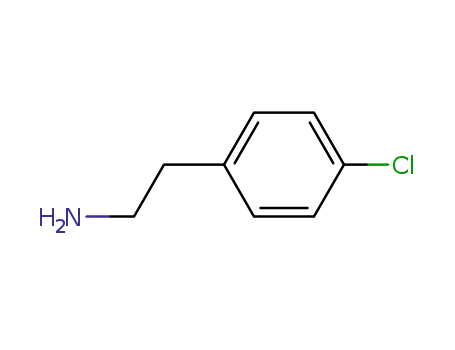
4-chlorophenylethylamine
| Conditions | Yield |
|---|---|
|
With aluminium trichloride; for 12h; Yield given. Title compound not separated from byproducts; Heating; 2 equivalents of AlCl3;
|
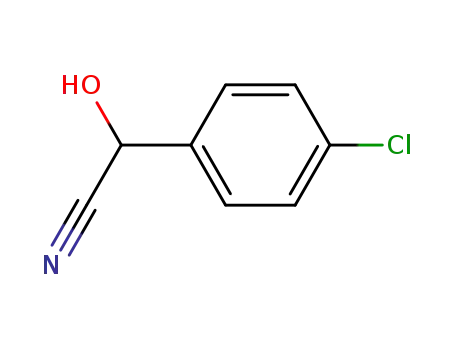
4-chloromandelonitrile
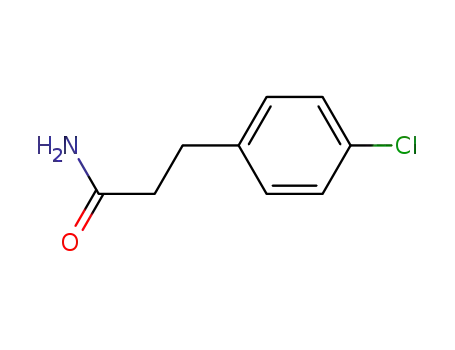
3-(4-chlorophenyl)propionic amide
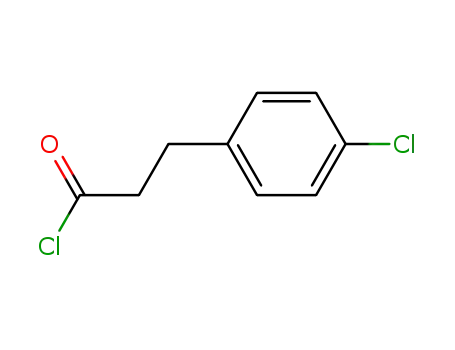
3-(4-chlorophenyl)propanoyl chloride
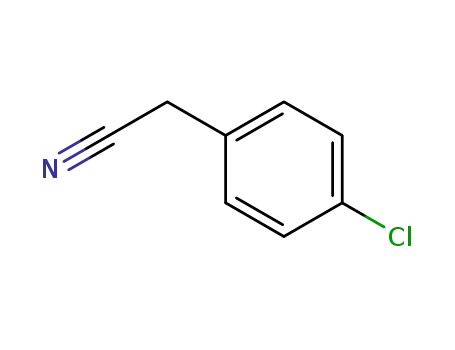
p-chlorobenzyl cyanide
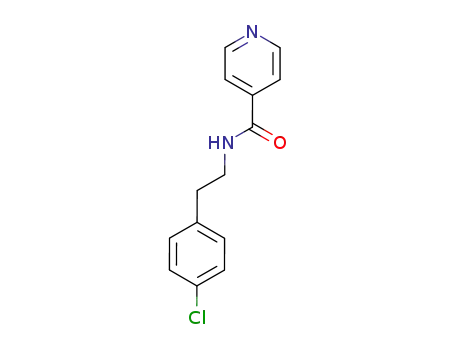
isonicotinic acid-(4-chloro-phenethylamide)
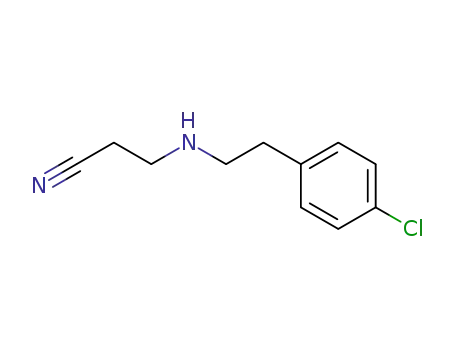
N-(4-chloro-phenethyl)-β-alanine nitrile
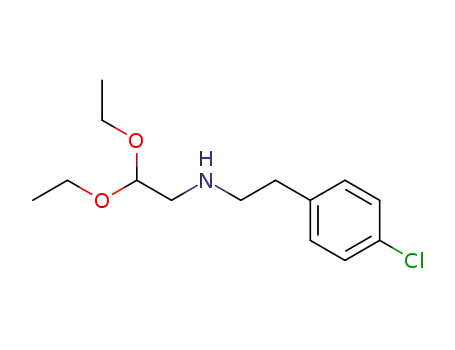
(4-chloro-phenethylamino)-acetaldehyde diethylacetal
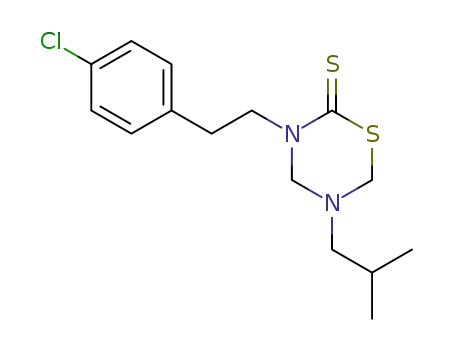
3-(4-chloro-phenethyl)-5-isobutyl-[1,3,5]thiadiazinane-2-thione
CAS:38083-17-9
CAS:120011-70-3
CAS:13078-79-0
CAS:1074-16-4