- Language:English
- English

CasNo: 10269-01-9
Molecular Formula: C7H8BrN
Appearance: clear yellow liquid
|
Chemical Properties |
clear yellow liquid |
|
Uses |
3-Bromobenzylamine, a potential inhibitor of the host ACE2 protein, is an important raw material and intermediate used in organic synthesis, pharmaceuticals, agro chemicals, dyestuff. |
InChI:InChI=1/C7H8BrN/c8-7-3-1-2-6(4-7)5-9/h1-4H,5,9H2
Novel benzo-anellated furo- and pyrrolo[...
The binary hydride, diisobutylaluminum b...
This work includes an effective comparis...
The antimicrobial activity of the compounds was evaluated against several bacterial and fungal strains, and it was found that some of the compounds exhibited significant activity against certain strains. Overall, the authors conclude that the 3-substituted benzylamines synthesized in this study have potential as antimicrobial agents.
For this, the thiolactone end-groups of the oligomers were first ring-opened by using 3-bromobenzylamine.
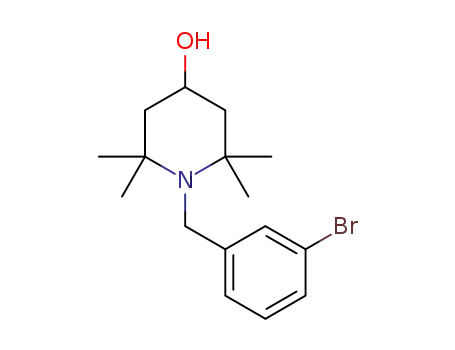
N-m-bromobenzyl-2,2,6,6-tetramethylpiperidin-4-ol

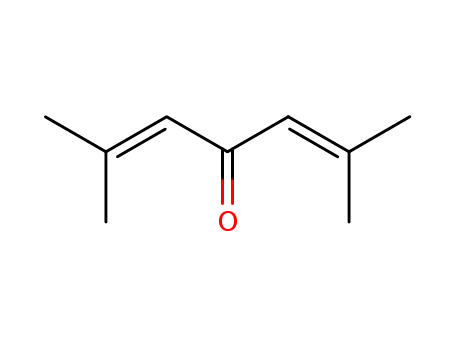
phorone

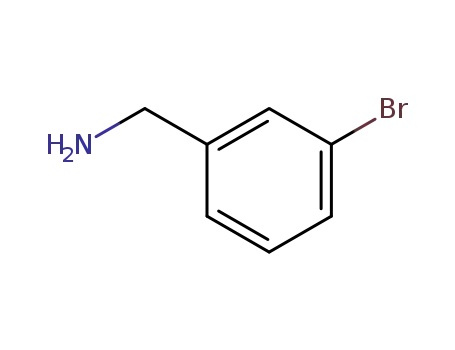
3-bromobenzylamine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: C20H29ClO2P2Pt; 1-Phenylbut-1-en-3-one; sodium hydroxide / toluene / 4 h / 105 °C
2: hydrogenchloride / 5 h / 105 °C
With hydrogenchloride; C20H29ClO2P2Pt; 1-Phenylbut-1-en-3-one; sodium hydroxide; In toluene;
|
C16H22BrNO


phorone


3-bromobenzylamine
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; at 105 ℃; for 5h;
|
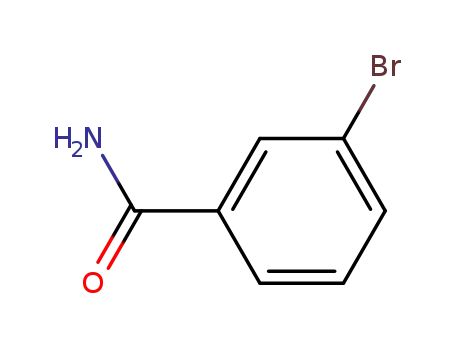
3-bromobenzamide
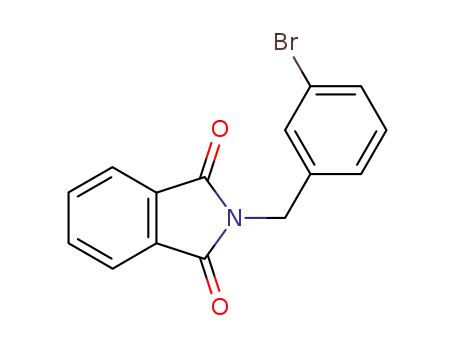
2-(3-bromobenzyl)isoindoline-1,3-dione
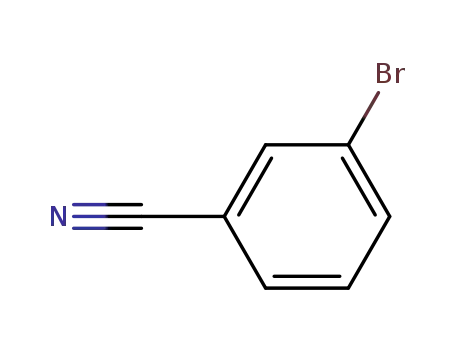
3-cyanobromobenzene
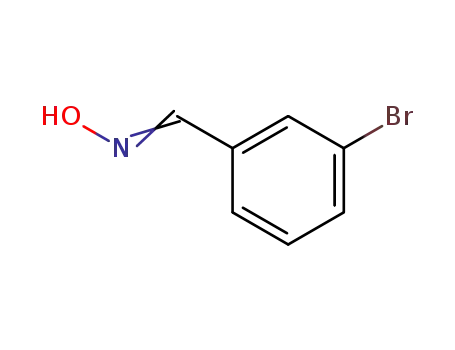
3-bromobenzaldehyde oxime
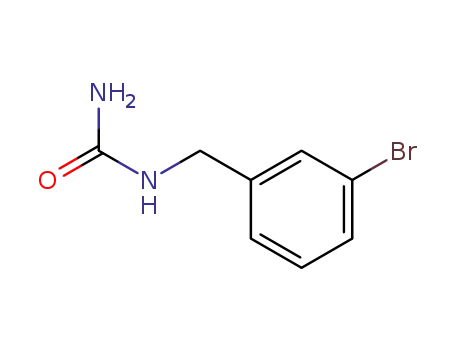
(3-bromo-benzyl)-urea
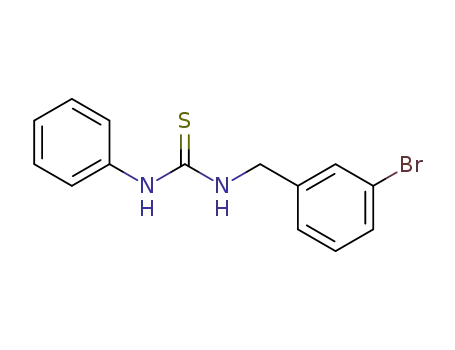
N-(3-bromo-benzyl)-N'-phenyl-thiourea

benzylidene-(3-bromo-benzyl)-amine
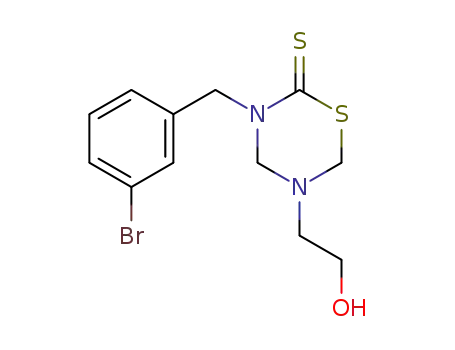
3-(3-bromo-benzyl)-5-(2-hydroxy-ethyl)-[1,3,5]thiadiazinane-2-thione
CAS:1953-04-4
CAS:20826-04-4
CAS:3959-07-7
CAS:65185-58-2