- Language:English
- English


CasNo: 65185-58-2
Molecular Formula: C8H10BrN
Appearance: clear colorless to slightly yellow liquid
|
Chemical Properties |
Clear colorless to slightly yellow liquid |
|
Uses |
2-(2-Bromophenyl)ethylamine is used as a pharmaceutical intermediate. In some studies, a successful selective synthesis of indolines was performed via intramolecular C—N the bond-forming reaction of 2-bromophenethylamine derivatives . |
|
General Description |
May darken in storage |
InChI:InChI=1/C8H10BrN/c9-8-4-2-1-3-7(8)5-6-10/h1-4H,5-6,10H2/p+1
The binary hydride, diisobutylaluminum b...
The reaction of 2-bromophenethylamine with 2-bromophenylboronic acid in the presence of 4 mol% of Pd(OAc) 2 and 8 mol% of 1 at 110 C for 24 h afforded 11 in 53% isolated yield ...
Using this method tetrahydroisoquinolines 4–8 were formed by reaction of 2-bromophenethylamine (3) with commercial acrylates using either the Pd(OAc) 2 /PPh 3 or the Pd(OAc) 2 /…
The one-pot synthesis of N-arylated indoles was also performed from 2-bromophenethylamine via intramolecular cross-coupling and N-arylation with aryl bromides. …
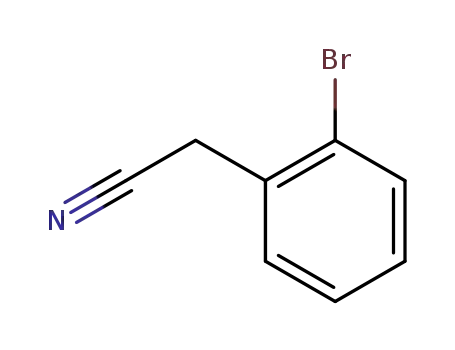
2-(2-bromophenyl)acetonitrile

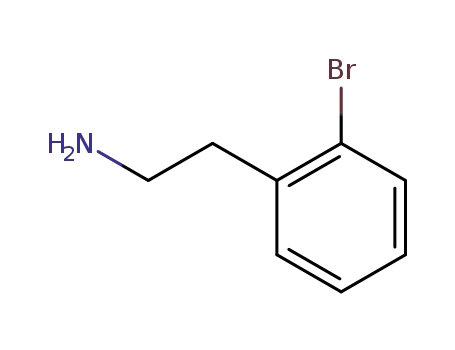
2-(2-bromophenyl)ethanamine
| Conditions | Yield |
|---|---|
|
2-(2-bromophenyl)acetonitrile; With borane tetrahydrofuran; In tetrahydrofuran; for 24h; Reflux;
With methanol; sulfuric acid; In tetrahydrofuran; water; at 20 ℃; for 1h;
With sodium hydroxide; In tetrahydrofuran; water;
|
94% |
|
With potassium hydroxide;
|
93% |
|
With lithium aluminium tetrahydride; aluminium trichloride; In diethyl ether; for 18h;
|
90% |
|
With lithium aluminium tetrahydride; aluminium trichloride; In diethyl ether; 1.) 0 deg C, 10 min, 2.) room temp., 2 h;
|
87% |
|
With sodium tetrahydroborate; In water; at 100 ℃; for 12h; pH=5.5;
|
87% |
|
With lithium aluminium tetrahydride; aluminium trichloride; In diethyl ether;
|
85% |
|
2-(2-bromophenyl)acetonitrile; With borane-THF; In tetrahydrofuran; at 0 ℃; for 24.5h; Reflux;
for 4h; Reflux;
|
85.2% |
|
2-(2-bromophenyl)acetonitrile; With borane-THF; In tetrahydrofuran; at 80 ℃; for 24h; Cooling with ice;
With hydrogenchloride; In tetrahydrofuran; methanol; water; at 80 ℃; for 4h;
|
85.2% |
|
With lithium aluminium tetrahydride;
|
82% |
|
With lithium borohydride; diisopropopylaminoborane; In tetrahydrofuran; for 18h; Reflux;
|
80% |
|
With diisobutylaluminum borohydride; In tetrahydrofuran; at 25 ℃; for 1h; Inert atmosphere;
|
74% |
|
With borane-THF; In tetrahydrofuran; at 0 - 20 ℃;
|
50% |
|
With lithium aluminium tetrahydride;
|
|
|
With sodium hydroxide; In tetrahydrofuran; water; ethyl acetate;
|
600 mg (55%) |
|
With sodium hydroxide; In tetrahydrofuran; water; ethyl acetate;
|
600 mg (55%) |
|
With aluminum (III) chloride; lithium aluminium tetrahydride; In diethyl ether; at 0 ℃; for 3h; Inert atmosphere;
|
|
|
With sodium tetrahydroborate; nickel dichloride;
|
|
|
With sodium tetrahydroborate; nickel(II) chloride hexahydrate; In methanol; at 0 - 20 ℃; for 1.5h;
|
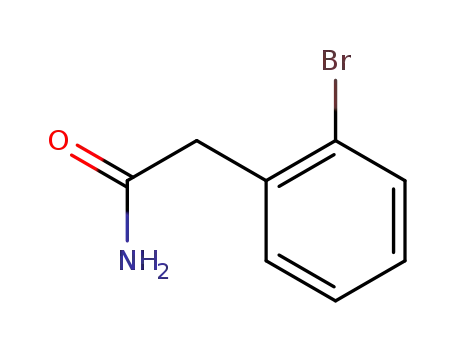
2-(2-bromophenyl)acetamide

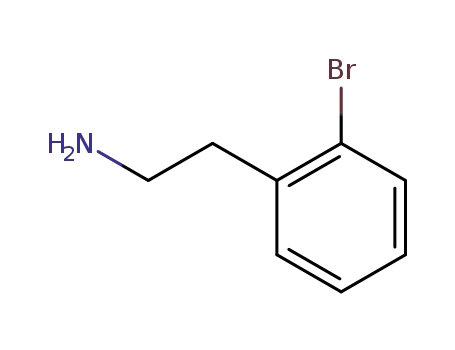
2-(2-bromophenyl)ethanamine
| Conditions | Yield |
|---|---|
|
With dimethylsulfide borane complex; In tetrahydrofuran; at 0 - 20 ℃;
|
70.4% |
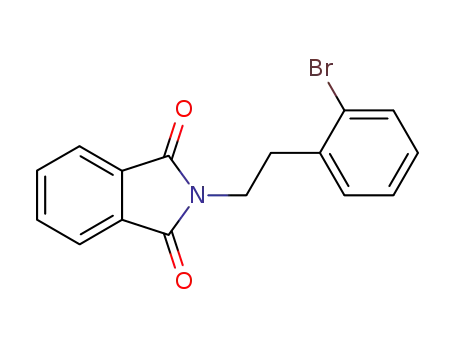
N-<β-(2-bromophenyl)ethyl>phthalimide
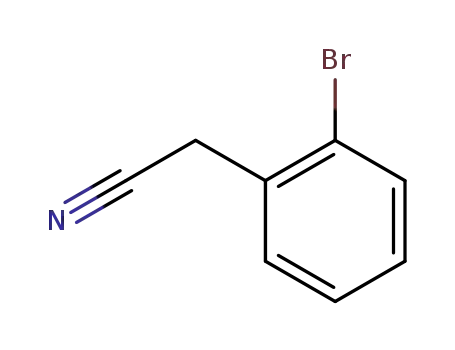
2-(2-bromophenyl)acetonitrile
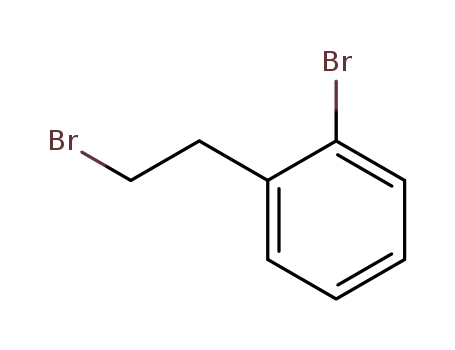
1-bromo-2-(2-bromoethyl)benzene
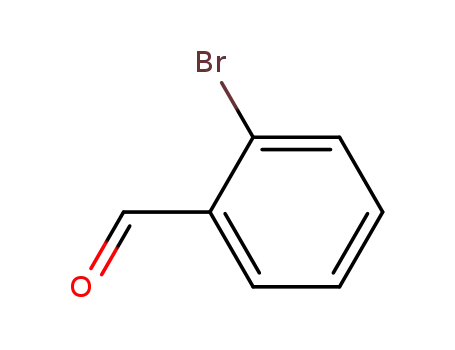
ortho-bromobenzaldehyde
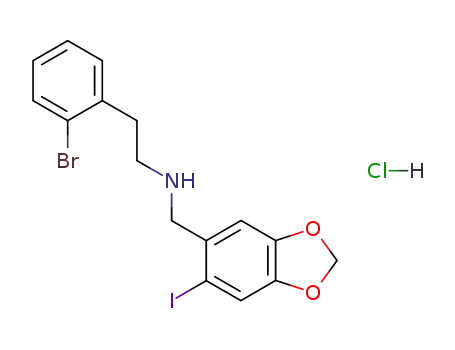
[2-(2-Bromo-phenyl)-ethyl]-(6-iodo-benzo[1,3]dioxol-5-ylmethyl)-amine; hydrochloride
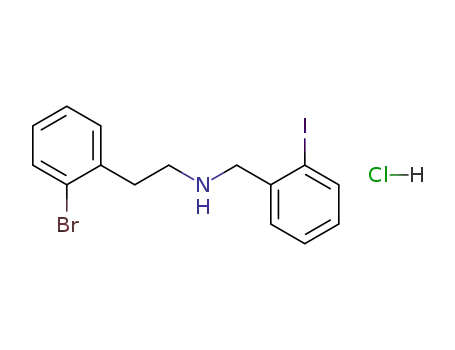
[2-(2-Bromo-phenyl)-ethyl]-(2-iodo-benzyl)-amine; hydrochloride
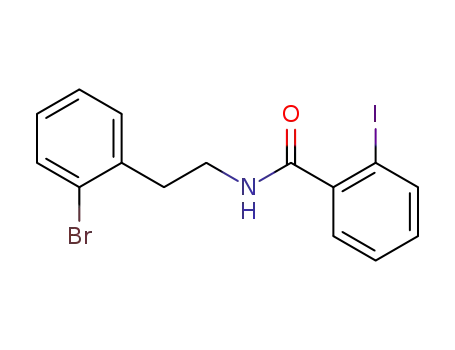
N-[2-(2-Bromo-phenyl)-ethyl]-2-iodo-benzamide
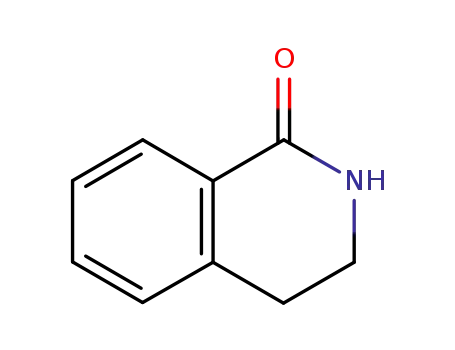
3,4-dihydroisoquinolin-1(2H)-one
CAS:1953-04-4
CAS:20826-04-4
CAS:10269-01-9
CAS:58971-11-2