- Language:English
- English
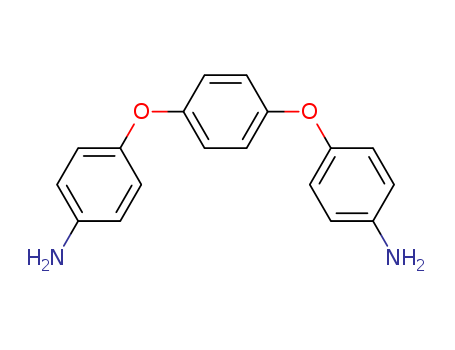

CasNo: 3491-12-1
Molecular Formula: C18H16N2O2
|
Uses |
1,4-bis(4-aminophenoxy)benzene be used for preparation polyimide and epoxy resin material. 1,4-Bis(4-aminophenoxy)benzene (TPE-Q ), C18H16N2O2, is a precusor for the synthesis of polyimides. The molecule is located on a crystallographic inversion center and the terminal aminophenoxy rings are almost perpendicular to the central benzene ring with a dihedral angle of 85.40 (4)°. The molecular conformation is stabilized by N-H…O and N-H…N intermolecular hydrogen-bonding interactions. |
InChI:InChI=1/C18H16N2O2/c19-13-1-5-15(6-2-13)21-17-9-11-18(12-10-17)22-16-7-3-14(20)4-8-16/h1-12H,19-20H2
The copolyimides were prepared with PMDA as an anhydride monomer, ODA as an amine monomer with the addition of 2,2-bis[4-(4-aminephenoxy)phenyl]propane, 1,4-bis(4-aminophenoxy)benzene, or 1,3-bis(4-aminophenoxy)benzene as another amine monomer.
In order to make clear how the numbers o...
A series of constitutional isomers conta...
In this paper, we report the synthesis and characterization of isomeric polyimides derived from a new diamine monomer, 1,4-bis(4-amino-3-trifluoromethylphenoxy)benzene, and a known analog 1,4-bis(4-amino-2-trifluoromethylphenoxy)benzene. The isomeric ditrifluoromethyl substituted aromatic diamine, 1,4-bis(4-amino-3-trifluoromethylphenoxy)benzene (2), and a known analog 1,4-bis(4-amino-2-trifluoromethylphenoxy)benzene (3) were synthesized.
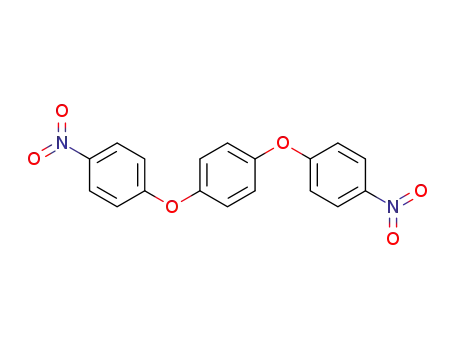
1,4'-bis(4-nitrophenoxy)benzene

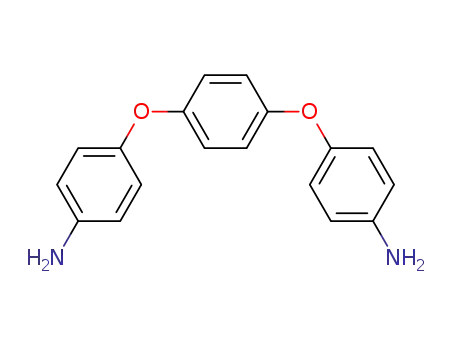
1,4-bis(4-aminophenoxy)benzene
| Conditions | Yield |
|---|---|
|
1,4'-bis(4-nitrophenoxy)benzene; With 5%-palladium/activated carbon; In ethanol; at 50 ℃; for 0.5h;
With hydrazine hydrate; In ethanol; for 5.5h; Reflux;
|
90% |
|
With iron(III) chloride hexahydrate; pyrographite; In ethanol; for 6h; Reflux;
|
85% |
|
With palladium 10% on activated carbon; hydrogen; In methanol; at 20 ℃; Inert atmosphere; Schlenk technique;
|
69% |
|
With hydrogenchloride; tin;
|
|
|
With hydrogen; nickel;
|
|
|
With NH2-NH2/FeCl3.H2O/active-C; In ethanol; for 6h; Reflux;
|
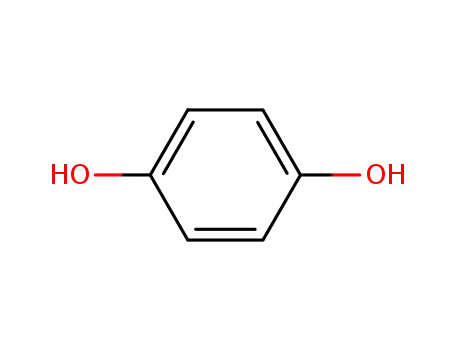
hydroquinone


1,4-bis(4-aminophenoxy)benzene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: potassium carbonate / N,N-dimethyl-formamide / 8 h / 145 - 150 °C / Inert atmosphere
2: iron(III) chloride hexahydrate; pyrographite / ethanol / 6 h / Reflux
With iron(III) chloride hexahydrate; potassium carbonate; pyrographite; In ethanol; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1: Inert atmosphere; Schlenk technique
2: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / Inert atmosphere; Schlenk technique
With palladium 10% on activated carbon; hydrogen; In methanol;
|
|
|
Multi-step reaction with 2 steps
1: potassium carbonate / N,N-dimethyl-formamide / 8 h / 140 °C
2: NH2-NH2/FeCl3.H2O/active-C / ethanol / 6 h / Reflux
With potassium carbonate; In ethanol; N,N-dimethyl-formamide;
|

1,4'-bis(4-nitrophenoxy)benzene
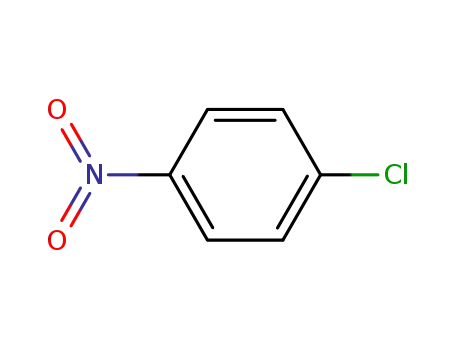
4-chlorobenzonitrile

hydroquinone
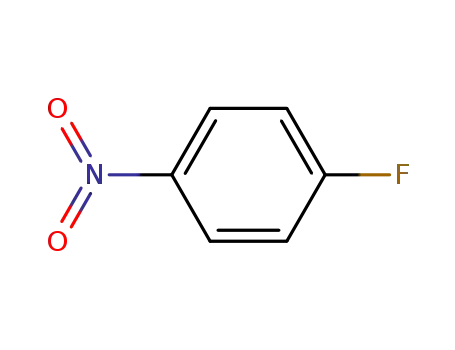
4-Fluoronitrobenzene
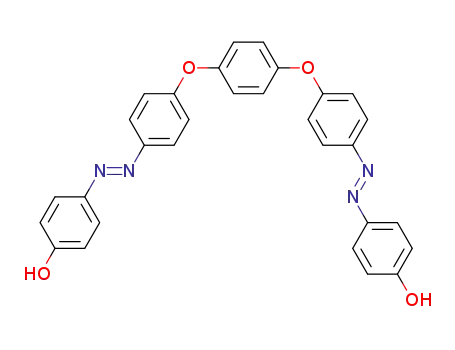
1,4-bis-[4-(4-hydroxy-phenylazo)-phenoxy]-benzene
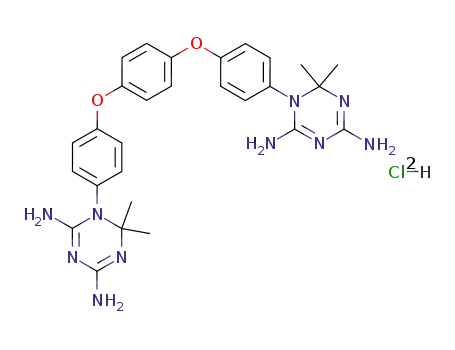
C28H32N10O2*2ClH
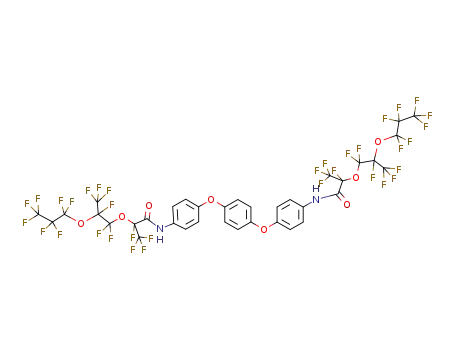
C36H14F34N2O8
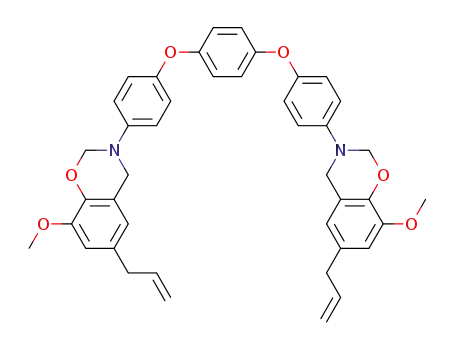
C42H40N2O6
CAS:138071-82-6
CAS:38083-17-9