- Language:English
- English
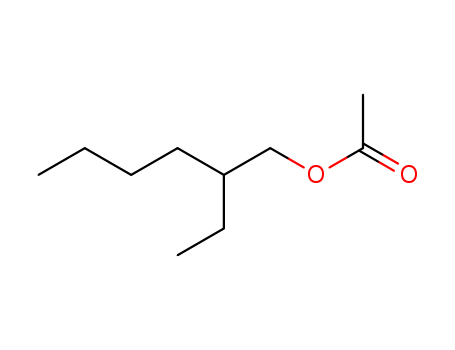

CasNo: 103-09-3
Molecular Formula: C10H20O2
Appearance: clear colorless liquid
|
Synthesis Reference(s) |
Synthetic Communications, 20, p. 125, 1990 DOI: 10.1080/00397919008054623Tetrahedron Letters, 23, p. 5407, 1982 DOI: 10.1016/0040-4039(82)80142-1 |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
2-Ethylhexyl acetate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Contact with strong oxidizers may cause vigorous reaction [USCG, 1999]. |
|
Health Hazard |
Prolonged skin contact may cause irritation. |
|
Fire Hazard |
Special Hazards of Combustion Products: Irritating vapors and toxic gases, such as carbon monoxide, may be formed when involved in fire. |
|
Flammability and Explosibility |
Nonflammable |
|
Safety Profile |
Moderately toxic by ingestion. Askin and eye irritant. Flammable when exposed to heat orflame; can react with oxidizing materials. To fight fire, usefoam, CO2, dry chemical. When heated to decompositionit emits acrid smoke and irritating fumes. |
|
Definition |
ChEBI: An acetate ester that is hexyl acetate substituted by an ethyl group at position 2. |
|
General Description |
A water-white liquid. Insoluble in water and is less dense than water. Flash point 180°F. Used as a solvent and in perfumes. Liquid or vapors may irritate skin and eyes. Vapors have a fruity, pleasant odor. |
InChI:InChI=1/C10H20O2/c1-4-6-7-10(5-2)8-12-9(3)11/h10H,4-8H2,1-3H3/t10-/m1/s1
Polystyrene (PS)-supported 1-(propyl-3-s...
Deep eutectic solvents (DES) containing ...
In present study, the application of ste...
Alcohols and phenols were efficiently ac...
A gold nanoparticle (AuNP)-supported gad...
Efficient esterification of primary and ...
A highly efficient and selective method ...
A facile strategy for the synthesis of a...
In order to explore a new application fi...
Alcohols and phenols are converted to es...
A novel metal-organic framework, Cu-BDC ...
The Br?nsted acidic ionic liquid 1-(prop...
Acetylation and formylation reactions of...
In the present study, we have synthesize...
A general formamide-catalyzed protocol f...
The invention relates to a sun-screening...
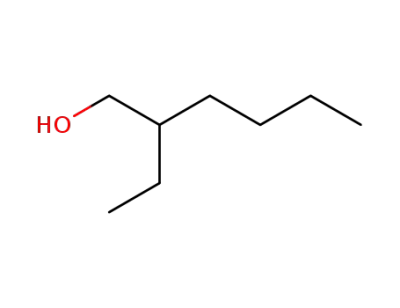
2-Ethylhexyl alcohol

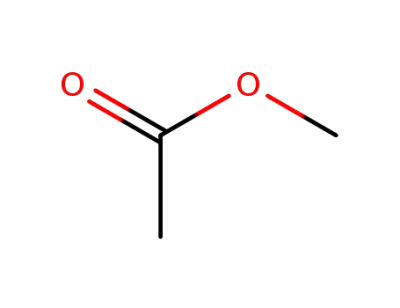
acetic acid methyl ester

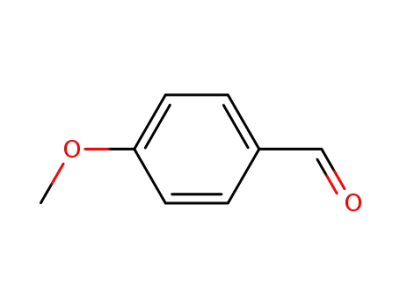
4-methoxy-benzaldehyde

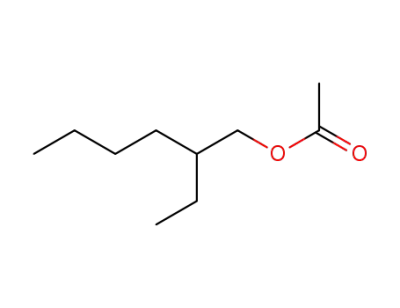
2-ethylhexyl acetate

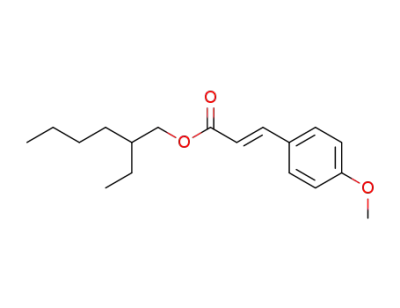
2-ethylhexyl methoxycinnamate
| Conditions | Yield |
|---|---|
|
2-Ethylhexyl alcohol; acetic acid methyl ester; 4-methoxy-benzaldehyde;
With
sodium methylate;
at 20 - 100 ℃;
for 3.33333h;
under 45.0045 Torr;
With
sulfuric acid; toluene-4-sulfonic acid;
In
water;
at 100 ℃;
for 0.25h;
|
91.5% |
|
2-Ethylhexyl alcohol; acetic acid methyl ester; 4-methoxy-benzaldehyde;
With
sodium methylate;
at 20 - 100 ℃;
for 3.33333h;
under 45.0045 Torr;
With
sulfuric acid;
In
water;
at 100 - 150 ℃;
for 2.25h;
|
90% |
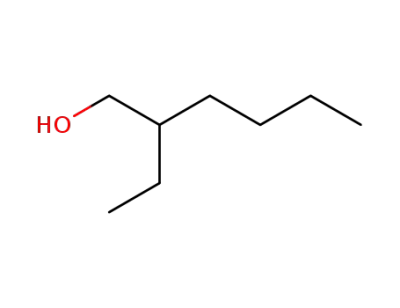
2-Ethylhexyl alcohol


acetic acid methyl ester

methanol


2-ethylhexyl acetate
| Conditions | Yield |
|---|---|
|
With
NKC-9;
at 80 ℃;
for 3h;
Reagent/catalyst;
Temperature;
Catalytic behavior;
|
90.9% |
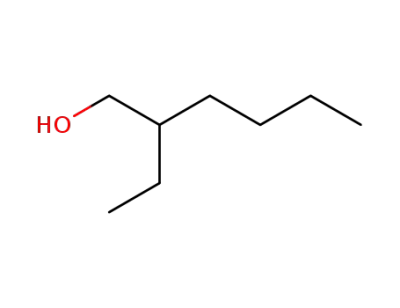
2-Ethylhexyl alcohol
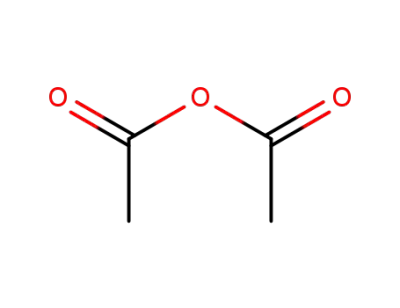
acetic anhydride
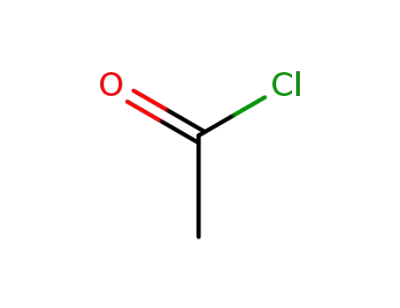
acetyl chloride
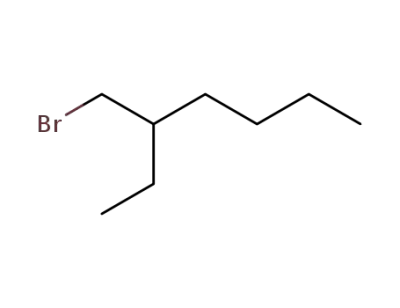
3-bromomethylheptane
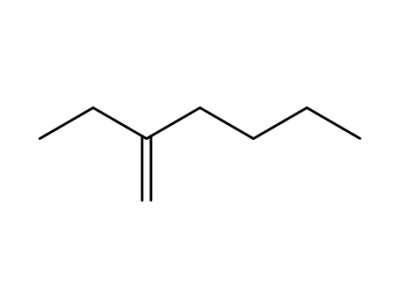
2-ethyl-1-hexene
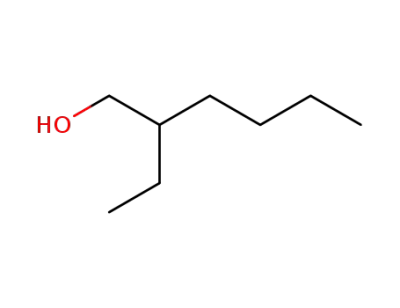
2-Ethylhexyl alcohol
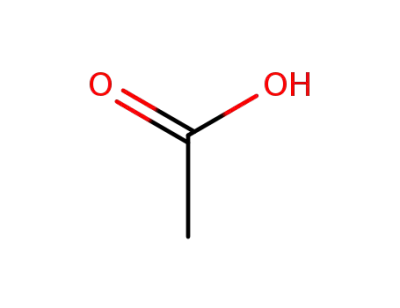
acetic acid
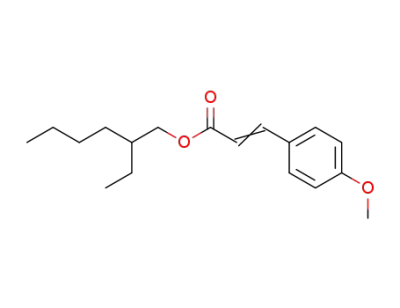
2-propenoic acid, 3-(4-methoxyphenyl)-, 2-ethylhexyl ester
CAS:138071-82-6
CAS:57280-22-5
CAS:9004-61-9
CAS:1313407-40-7