- Language:English
- English

CasNo: 103909-75-7
Molecular Formula: C26H42O4
|
Brand name |
Prezios (Chugai Pharmaceutical Co., Ltd., Japan);Prezios, Oxarol. |
InChI:InChI=1/C26H42O4/c1-17-20(15-21(27)16-24(17)28)9-8-19-7-6-12-26(5)22(10-11-23(19)26)18(2)30-14-13-25(3,4)29/h8-9,18,21-24,27-29H,1,6-7,10-16H2,2-5H3/b19-8+,20-9-/t18-,21+,22+,23-,24-,26+/m0/s1
Maxacalcitol, the 22-oxa-derivative of 1...
Synthesis of two tritiated 1α,25-dihydlo...
The stereoselective synthesis of 22-oxac...
The invention belongs to the field of ph...
The present invention provides a new met...
Maxacalcitol, the 22-oxa-derivative of 1...
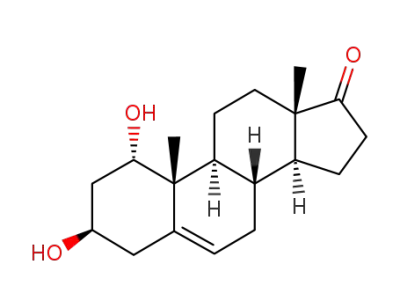
1α,3β-dihydroxy-androst-5-en-17-one

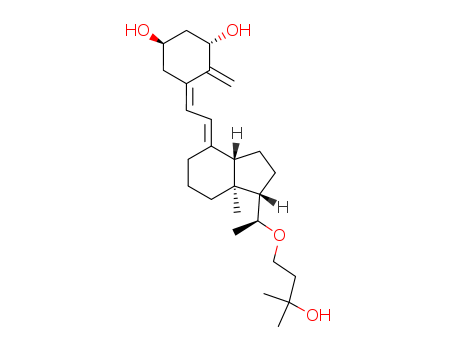
maxacalcitol
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 11 steps
1.1: 1H-imidazole / N,N-dimethyl-formamide / 6 h / 20 - 125 °C / Large scale
2.1: potassium tert-butylate / tetrahydrofuran / 4 h / 25 - 30 °C / Large scale
3.1: tetrahydrofuran / 4 h / 20 - 45 °C / Large scale
4.1: sodium hydroxide; dihydrogen peroxide / 1 h / 25 °C / Large scale
5.1: sodium hydride / tetrahydrofuran; mineral oil / 20 °C / Large scale
5.2: 1 h / 20 °C / Reflux; Large scale
6.1: L-Selectride / tetrahydrofuran / 1 h / Reflux; Large scale
7.1: N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) / n-heptane / 0.25 h / 78 °C
8.1: 2,4,6-trimethyl-pyridine / toluene / 2.5 h / 110 °C / Large scale
9.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 9 h / 66 °C
10.1: tetrahydrofuran / 7 h / 10 °C / Irradiation
11.1: tetrahydrofuran / 85 h / 25 °C
With
1H-imidazole; 2,4,6-trimethyl-pyridine; N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile); potassium tert-butylate; tetrabutyl ammonium fluoride; dihydrogen peroxide; L-Selectride; sodium hydride; sodium hydroxide;
In
tetrahydrofuran; n-heptane; N,N-dimethyl-formamide; toluene; mineral oil;
2.1: |Wittig Olefination;
|
|
|
Multi-step reaction with 12 steps
1.1: 1H-imidazole / N,N-dimethyl-formamide / 6 h / 20 - 125 °C / Large scale
2.1: potassium tert-butylate / tetrahydrofuran / 4 h / 25 - 30 °C / Large scale
3.1: tetrahydrofuran / 4 h / 20 - 45 °C / Large scale
4.1: sodium hydroxide; dihydrogen peroxide / 1 h / 25 °C / Large scale
5.1: sodium hydride; 15-crown-5 / tetrahydrofuran; mineral oil / 6 h / 0 °C / Large scale
6.1: cerium(III) chloride / tetrahydrofuran / 0.5 h / -15 °C
6.2: 0.5 h / -15 °C
7.1: cerium(III) chloride / tetrahydrofuran / 0.5 h / -15 °C / Large scale
7.2: 0.5 h / -15 °C / Large scale
8.1: N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) / n-heptane / 0.25 h / 78 °C
9.1: 2,4,6-trimethyl-pyridine / toluene / 2.5 h / 110 °C / Large scale
10.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 9 h / 66 °C
11.1: tetrahydrofuran / 7 h / 10 °C / Irradiation
12.1: tetrahydrofuran / 85 h / 25 °C
With
1H-imidazole; 2,4,6-trimethyl-pyridine; N-Bromosuccinimide; cerium(III) chloride; 15-crown-5; 2,2'-azobis(isobutyronitrile); potassium tert-butylate; tetrabutyl ammonium fluoride; dihydrogen peroxide; sodium hydride; sodium hydroxide;
In
tetrahydrofuran; n-heptane; N,N-dimethyl-formamide; toluene; mineral oil;
2.1: |Wittig Olefination;
|

1α,3β-dihydroxy-androst-5-en-17-one

1α,25-dihydroxy-22-oxavitamin D3
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 7 steps
1: 94 percent / imidazole, 1-hydroxybenzotriazole / dimethylformamide / 72 h / 50 - 60 °C
2: 1.) NBS; 2.) γ-collidine / 1.) hexane, reflux, 1 h; 2.) xylene, reflux, 1 h
3: 64 percent / dimethylsulfoxide; tetrahydrofuran / Ambient temperature
4: 1.) 9-BBN; 2.) NaOH, H2O2 / 1.) THF, r.t., 16 h
5: 1.) NaH; 2.) O2, PdCl2, CuCl / 1.) xylene, reflux, 18 h; 2.) DMF-H2O, r.t. 19 h
6: 79 percent / tetrahydrofuran / 1 h / 0 °C
With
1H-imidazole; 2,4,6-trimethyl-pyridine; sodium hydroxide; N-Bromosuccinimide; 9-borabicyclo[3.3.1]nonane dimer; dihydrogen peroxide; oxygen; sodium hydride; benzotriazol-1-ol; copper(l) chloride; palladium dichloride;
In
tetrahydrofuran; dimethyl sulfoxide; N,N-dimethyl-formamide;
|
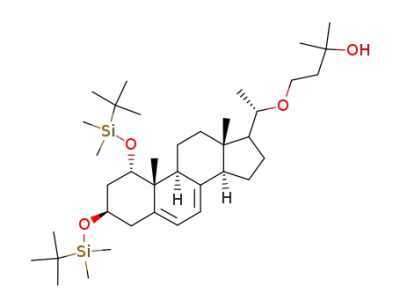
4-{(S)-1-[(1S,3R,9S,10R,13S,14R)-1,3-Bis-(tert-butyl-dimethyl-silanyloxy)-10,13-dimethyl-2,3,4,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]-ethoxy}-2-methyl-butan-2-ol

1α,3β-dihydroxy-androst-5-en-17-one
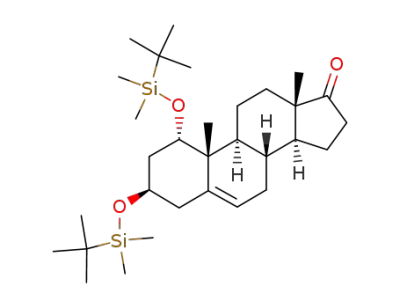
1α,3β-bis<(tert-butyldimethylsilyl)oxy>androst-5-en-17-one
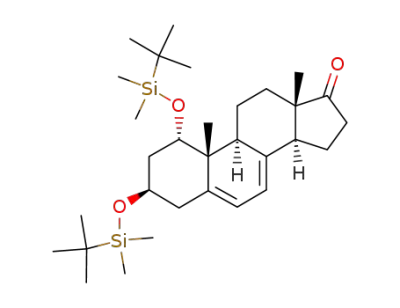
1α,3β-Bis(tert-butyldimethylsilyloxy)-17-oxoandrosta-5,7-diene
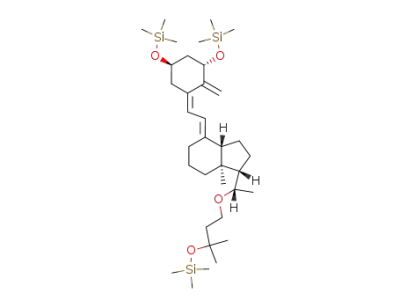
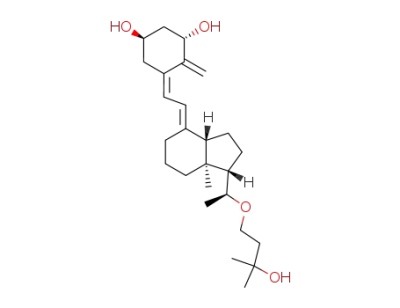
(1S,3aS,7aS)-7a-Methyl-4-[2-[(3S,5R)-2-methylene-3,5-bis-trimethylsilanyloxy-cyclohex-(Z)-ylidene]-eth-(E)-ylidene]-1-[(S)-1-(3-methyl-3-trimethylsilanyloxy-butoxy)-ethyl]-octahydro-indene
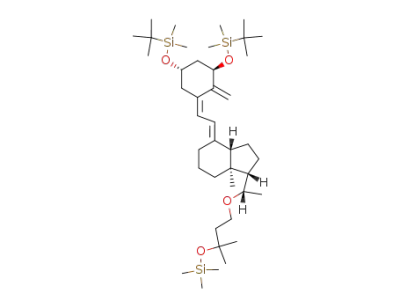
(5Z,7E,1R,3S,20S)-1,3-bis(tert-butyldimethylsilyloxy)-20-(3-methyl-3-trimethylsilyloxybutyloxy)-9,10-secopregna-5,7,10(19)-triene
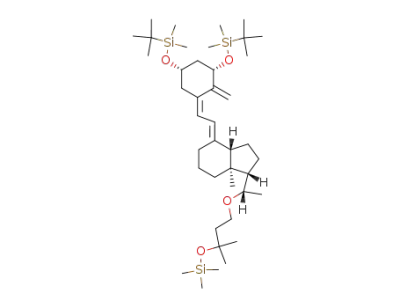
(5Z,7E,1S,3S,20S)-1,3-bis(tert-butyldimethylsilyloxy)-20-(3-methyl-3-trimethylsilyloxybutyloxy)-9,10-secopregna-5,7,10(19)-triene
CAS:138071-82-6
CAS:57280-22-5
CAS:156545-07-2
CAS:163217-09-2