- Language:English
- English
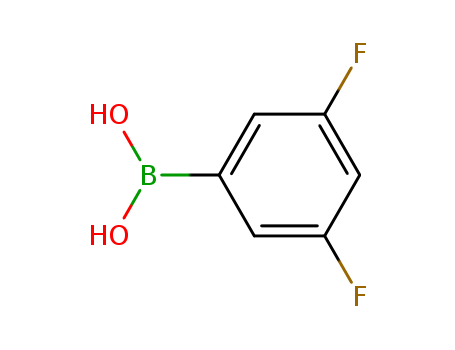

CasNo: 156545-07-2
Molecular Formula: C6H5BF2O2
Appearance: White to beige-yellowish powder
InChI:InChI=1/C13H11BF2O3/c15-12-10(14(17)18)6-7-11(13(12)16)19-8-9-4-2-1-3-5-9/h1-7,17-18H,8H2
In this work, we depict the synthesis an...
The invention provides a 3, 5-difluoroph...
The present invention relates to a compo...
The Suzuki-Miyaura reaction has become o...
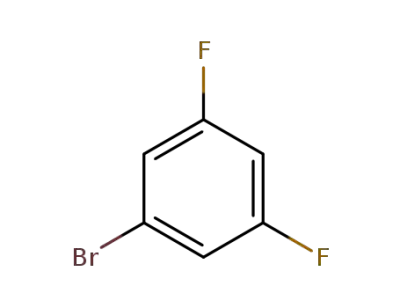
3,5-difluorobromobenzene

boric acid

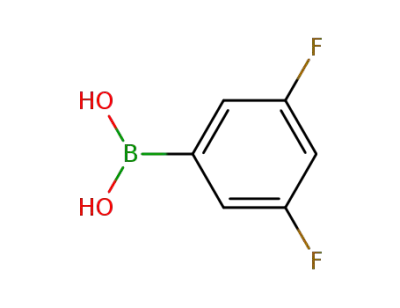
3,5-difluorophenylboronic acid
| Conditions | Yield |
|---|---|
|
3,5-difluorobromobenzene;
With
n-butyllithium;
In
tetrahydrofuran;
at -60 ℃;
for 2h;
Inert atmosphere;
boric acid;
In
tetrahydrofuran;
for 1h;
Temperature;
Inert atmosphere;
|
85.8% |
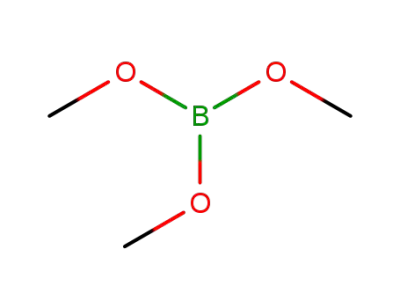
Trimethyl borate


3,5-difluorobromobenzene


3,5-difluorophenylboronic acid
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; magnesium;
In
tetrahydrofuran;
|
|
|
With
hydrogenchloride; tert.-butyl lithium;
In
diethyl ether; cyclohexane;
|
|
|
3,5-difluorobromobenzene;
With
magnesium;
In
tetrahydrofuran;
at 45 - 55 ℃;
Inert atmosphere;
Trimethyl borate;
In
tetrahydrofuran;
at -70 - 0 ℃;
Inert atmosphere;
With
hydrogenchloride; water;
In
tetrahydrofuran; ethyl acetate;
at 0 ℃;
|
|
|
3,5-difluorobromobenzene;
With
magnesium;
In
tetrahydrofuran;
at 40 ℃;
for 1h;
Inert atmosphere;
Cooling with ice;
Trimethyl borate;
In
tetrahydrofuran;
at 20 ℃;
for 1h;
Inert atmosphere;
Cooling with ice;
With
hydrogenchloride;
In
tetrahydrofuran; water;
at 20 ℃;
for 0.5h;
Inert atmosphere;
|
71.65 g |
|
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
|

3,5-difluorobromobenzene

Trimethyl borate
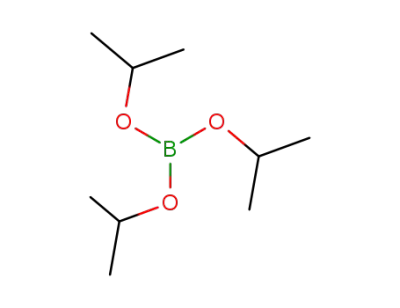
Triisopropyl borate
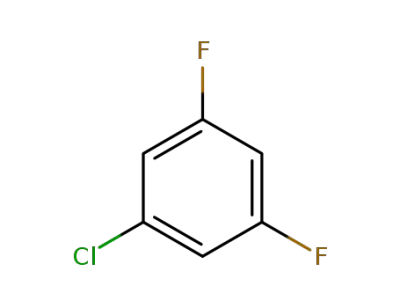
1-chloro-3,5-difluorobenzene

3,5-difluoro-4'-propylbiphenyl
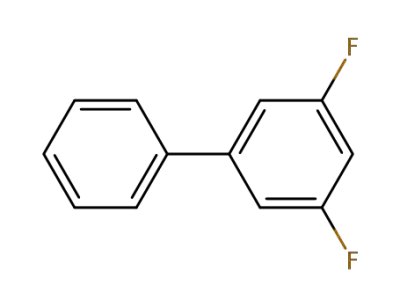
3,5-difluorobiphenyl
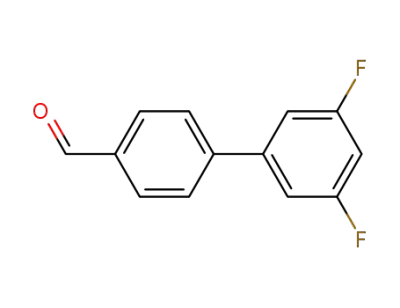
3',5'‐difluoro‐[1,1'‐biphenyl]‐4‐carbaldehyde
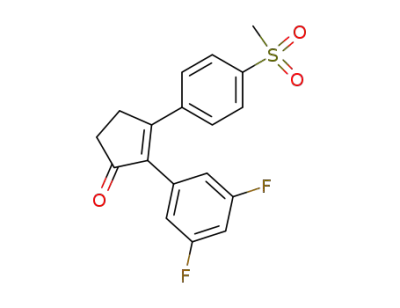
2-(3,5-difluorophenyl)-3-(4-(methylsulfonyl)phenyl)-2-cyclopenten-1-one
CAS:480424-84-8
CAS:108-59-8
CAS:103909-75-7