- Language:English
- English

CasNo: 605-23-2
Molecular Formula: C10H14N2O6
InChI:InChI=1/C10H14N2O6/c1-4-2-12(10(17)11-8(4)16)9-7(15)6(14)5(3-13)18-9/h2,5-7,9,13-15H,3H2,1H3,(H,11,16,17)/t5-,6-,7+,9-/m1/s1
1-β-D-Arabinofuranosylthymine (aThy; ara...
The present invention comprises novel an...
The oligodeoxyribonucleotides bearing 2,...
Compositions for topical use in herpes v...
A novel type of nucleotide analogues, th...
![2-((3aR,5R,6R,6aS)-6-Hydroxy-5-hydroxymethyl-2-imino-tetrahydro-furo[2,3-d]oxazol-3-ylmethyl)-acrylic acid ethyl ester; hydrobromide](/upload/2025/12/c4525fb9-7594-4956-a046-f7aa6bd2d1eb.png)
2-((3aR,5R,6R,6aS)-6-Hydroxy-5-hydroxymethyl-2-imino-tetrahydro-furo[2,3-d]oxazol-3-ylmethyl)-acrylic acid ethyl ester; hydrobromide

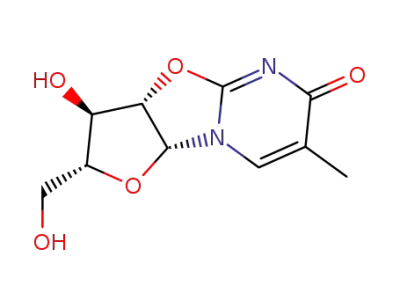
O-2,2'-cyclo-5-methyluridine

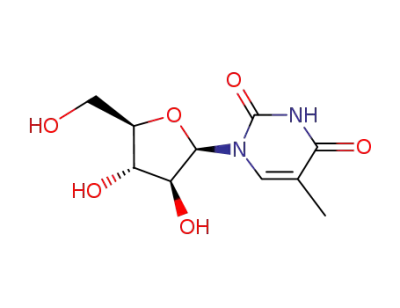
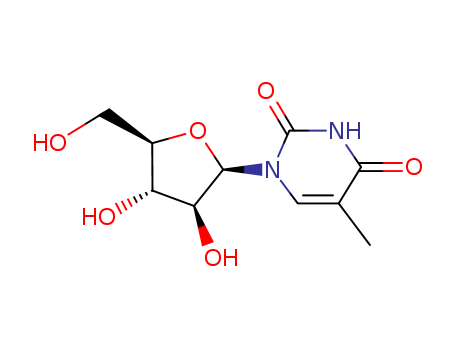
thymine arabinoside
| Conditions | Yield |
|---|---|
|
With
potassium tert-butylate;
In
tert-butyl alcohol;
at 25 ℃;
for 20h;
|
32% 14% |

5-Methyluridine


thymine arabinoside
| Conditions | Yield |
|---|---|
|
With
bis(phenyl) carbonate; copper; sodium hydrogencarbonate;
In
N,N-dimethyl-formamide;
at 140 - 150 ℃;
for 45h;
|
50% |
|
With
hydrogenchloride; O-acetylsalicyloyl chloride;
Yield given. Multistep reaction;
1.) nitromethane, 20 deg C, 15 h, 2.) water, dioxane, 100 deg C, 2.5 h;
|
|
|
With
hydrogenchloride; O-acetylsalicyloyl chloride;
Yield given. Multistep reaction;
2.) water, dioxane;
|
|
|
Multi-step reaction with 2 steps
1: pyridine
2: aufeinanderfolgende Behandlung des Reaktionsprodukts mit methanol.NH3 und mit wss.H2SO4
With
pyridine;
|
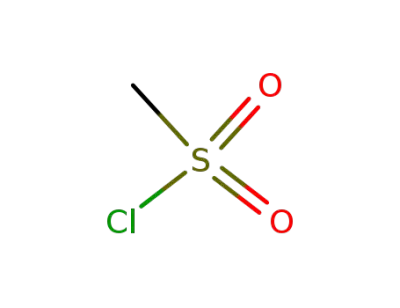
methanesulfonyl chloride

1-(5'-O-trityl-β-D-ribofuranosyl)thymine

O-2,2'-cyclo-5-methyluridine
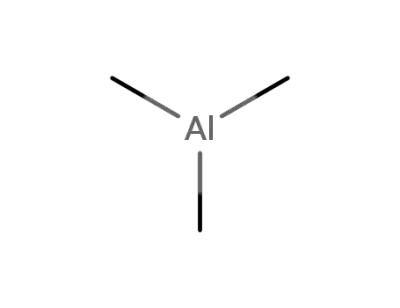
trimethylaluminum
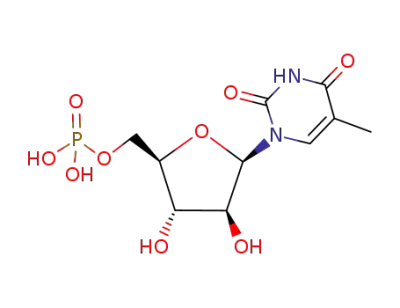
Phosphoric acid mono-[(2R,3S,4S,5R)-3,4-dihydroxy-5-(5-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-tetrahydro-furan-2-ylmethyl] ester
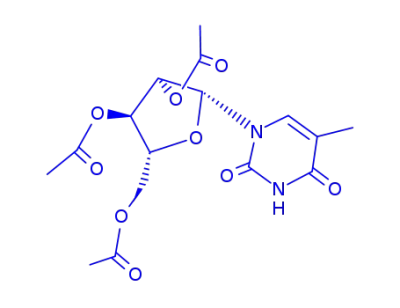
1-(Tri-O-acetyl-β-D-arabinofuranosyl)-thymin
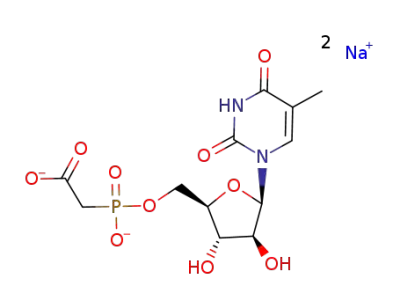
Sodium; {[(2R,3S,4S,5R)-3,4-dihydroxy-5-(5-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-tetrahydro-furan-2-ylmethoxy]-hydroxy-phosphoryl}-acetate
CAS:138071-82-6
CAS:38083-17-9
CAS:104121-92-8
CAS:52688-08-1