- Language:English
- English
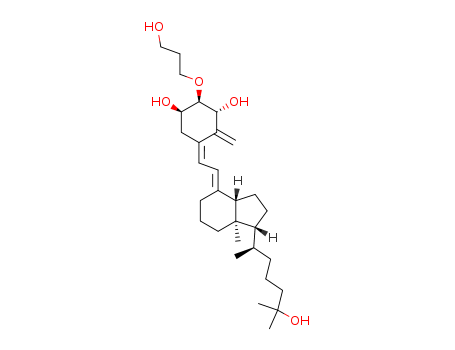

CasNo: 104121-92-8
Molecular Formula: C30H50O5
|
Synthesis |
The biomimetic vitamin D3 analog synthesis that was recently disclosed, based on an earlier reported route for the commercial synthesis of alfacalcidol, will be discussed here.An Oppenauer oxidation converted commercially available cholesterol 141 to enone 142 in 80% yield. A second oxidation event with DDQ provided dienone 143 in 75% yield. Treatment of 143 with sodium ethoxide in ethanol triggered migration of the enone double bond into the B-ring, giving olefin 144 in 53% yield. Stereospecific reduction of ketone 144 with sodium borohydride gave alcohol 145 in 53% yield, which was then immediately protected as the corresponding acetate with acetic anhydride to furnish 146. Next, further dehydrogenation of the B-ring was accomplished using radical bromination of the olefin within 146 through the use of NBS and catalytic AIBN, followed by elimination with collidine. A subsequent saponification step ultimately gave rise to the key diene 147. Next, in order to selectively epoxidize the A-ring olefin, a unique ‘protection’ strategy was employed using phenyl- 1,2,4-triazole-3,5-dione (PTAD). Diels–Alder reaction between diene 147 and PTAD produced cycloadduct 148 in 80% overall yield from acetate 146. Protection of the alcohol as the corresponding TBS ether preceeded a regio- and stereospecific epoxidation with m-CPBA to afford 1,2a-epoxide 150 in 78% yield. Diels–Alder adduct 150 was then subjected to thermal conditions to affect a retro-[ 4+2] reaction to give diene 151. Fluoride-mediated removal of the TBS group prepared 3b-alcohol 152 in 95% yield. Subsequent ring-opening reaction with 1,3-propane diol in the presence of potassium t-butoxide, provided 3-hydroxy propoxy ether 153 in 29% yield. Microbial oxidation of intermediate 153 was accomplished using an Amycolata autotrophica ATCC 33796 culture to obtain eldecalcitol derivative 154 in 64% yield. Subjection of 154 to 400 watt light followed by thermolysis provided eldecalcitol (XII) in 29% yield. |
|
Definition |
ChEBI: A hydroxycalciol that is calcitriol with a 3-hydroxypropoxy group at position 2. |
|
Brand name |
Edirol |
InChI:InChI=1/C30H50O5/c1-20(9-6-15-29(3,4)34)24-13-14-25-22(10-7-16-30(24,25)5)11-12-23-19-26(32)28(27(33)21(23)2)35-18-8-17-31/h11-12,20,24-28,31-34H,2,6-10,13-19H2,1,3-5H3/b22-11+,23-12-/t20-,24-,25+,26+,27+,28+,30-/m1/s1
Industrial-scale synthesis of eldecalcit...
The invention relates to new intermediat...
PROBLEM TO BE SOLVED: To provide a novel...
In a reported procedure for the synthesi...
C54H106O5Si4

Eldecalcitol
| Conditions | Yield |
|---|---|
|
With
tetrabutyl ammonium fluoride;
In
tetrahydrofuran;
at 25 ℃;
|
89% |
![(1β,2α,3α,5Z,7E)-1,3-bis[(1,1-dimethylethyl)dimethylsilyloxy]-2-[3-[(1,1-dimethylethyl)dimethylsilyloxy]propoxy]-25-trimethylsilyloxy-9,10-secocholesta-5,7,10(19)-triene](/upload/2025/12/d5f50480-67f3-4226-bf27-f6e7a0469b8a.png)
(1β,2α,3α,5Z,7E)-1,3-bis[(1,1-dimethylethyl)dimethylsilyloxy]-2-[3-[(1,1-dimethylethyl)dimethylsilyloxy]propoxy]-25-trimethylsilyloxy-9,10-secocholesta-5,7,10(19)-triene


Eldecalcitol
| Conditions | Yield |
|---|---|
|
With
pyridine; hydrogen fluoride;
In
tetrahydrofuran;
at 20 ℃;
|
96% |

25-[(triethylsilyl)oxy]de-A,B-cholestan-8-one

[3R-(1Z,3β,4α,5α)]-[2-[3,5-bis[(1,1-dimethylethyl)dimethylsilyloxy]-4-[3-[(1,1-dimethylethyl)dimethylsilyloxy]propoxy]-2-methylenecyclohexylidene]ethyl]diphenylphosphine oxide
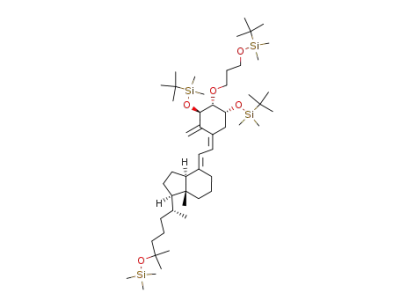
(1β,2α,3α,5Z,7E)-1,3-bis[(1,1-dimethylethyl)dimethylsilyloxy]-2-[3-[(1,1-dimethylethyl)dimethylsilyloxy]propoxy]-25-trimethylsilyloxy-9,10-secocholesta-5,7,10(19)-triene
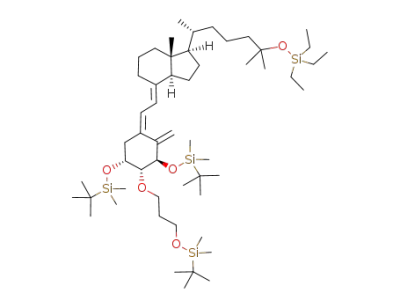
(5Z,7E)-(1R,2R,3R)-1,3-bis(tert-butyldimethylsilyloxy)-2-(3-tert-butyldimethylsilyloxypropoxy)-25-triethylsilyloxy-9,10-secocholesta-5,7,10(19)-triene
CAS:138071-82-6
CAS:57280-22-5
CAS:98-80-6
CAS:605-23-2