- Language:English
- English


CasNo: 5466-77-3
Molecular Formula: C18H26O3
Appearance: colourless or pale yellow liquid
|
Brand name |
Parsol (Roche); Neo Heliopan (H & R Florasynth); Escalol (ISP Van Dyk) Note—The International Cosmetic Ingredient (INCI) name for octinoxate is octyl methoxycinnamate. |
|
General Description |
Colorless to pale yellow viscous liquid. |
|
Air & Water Reactions |
Insoluble in water. |
|
Fire Hazard |
Flash point data for Octyl 4-methoxycinnamate are not available, however, Octyl 4-methoxycinnamate is probably combustible. |
InChI:InChI=1/C18H26O3/c1-4-6-7-8-16(5-2)21-18(19)14-11-15-9-12-17(20-3)13-10-15/h9-14,16H,4-8H2,1-3H3/b14-11+
Olefin metathesis has been widely explor...
p-Cresol as additive to the Grubbs secon...
A series of new unsymmetrical (XYC–1 typ...
We report for the first time cyclic phos...
The invention discloses a method for cat...
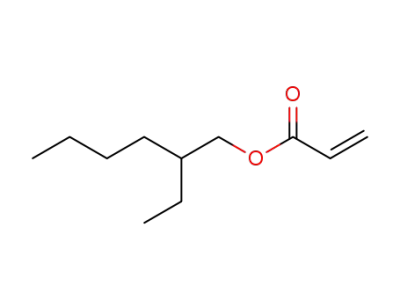
2-Ethylhexyl acrylate

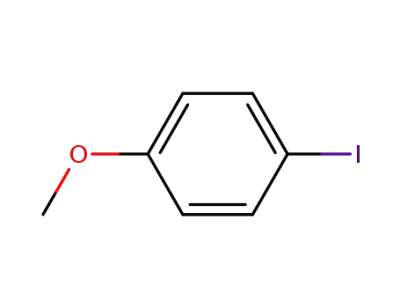
para-iodoanisole

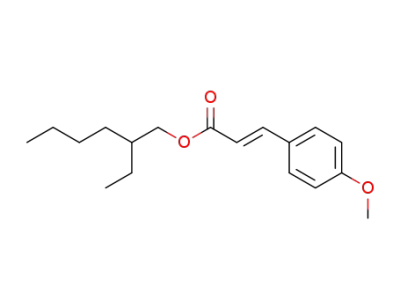
2-ethylhexyl methoxycinnamate
| Conditions | Yield |
|---|---|
|
With
tetrabutylammomium bromide; C32H26Cl2N8O4Pd2; potassium carbonate;
In
methanol; water;
for 0.416667h;
Reflux;
|
97% |
|
With
C43H54NO5P; palladium diacetate; triethylamine;
In
water;
at 40 ℃;
for 12h;
|
95% |
|
With
tributyl-amine; chloro-[2-(9-phenyl-1,10-phenanthrolin-2-yl)phenyl]palladium;
In
1-methyl-pyrrolidin-2-one;
at 140 ℃;
for 15h;
Inert atmosphere;
|
94% |
|
With
(Bis(tri-tert-butylphosphine)palladium(0)); SPGS-550-M; NOK; triethylamine;
In
water;
for 5h;
Reagent/catalyst;
Inert atmosphere;
Microwave irradiation;
Sealed tube;
|
93% |
|
With
triethylamine;
4,4'-dichlorobenzophenone oxime derived palladacycle;
In
1-methyl-pyrrolidin-2-one;
at 110 ℃;
for 22h;
|
85% |
|
With
dichloro[1,1'-bis(di-t-butylphosphino)ferrocene]palladium(II); pyridinium p-toluenesulfonate; triethylamine;
In
water;
at 20 ℃;
under 760.051 Torr;
Inert atmosphere;
Micellar solution;
|
84% |
|
With
1-methyl-pyrrolidin-2-one; triethylamine;
polymer-bound Pd(0) phosphine catalyst;
at 90 ℃;
|
|
|
With
acetic acid; diisopropylamine;
palladium on charcoal;
In
toluene;
|
|
|
With
potassium acetate;
In
1-methyl-pyrrolidin-2-one;
at 135 ℃;
for 24h;
|
60 %Chromat. |
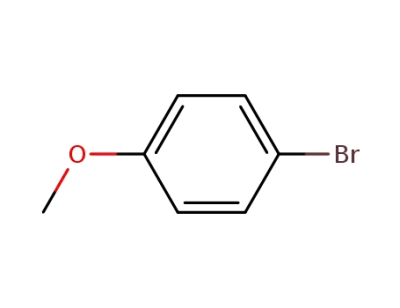
1-bromo-4-methoxy-benzene


2-Ethylhexyl acrylate


2-ethylhexyl methoxycinnamate
| Conditions | Yield |
|---|---|
|
With
tris(dibenzylideneacetone)dipalladium (0); tri-tert-butyl phosphine; Cy2NMe2;
In
1,4-dioxane;
at 20 ℃;
for 148h;
|
83% |
|
With
C21H21ClN4Pd; triethylamine;
In
1-methyl-pyrrolidin-2-one;
at 140 ℃;
for 8h;
|
73% |
|
With
sodium acetate;
|
71% |
|
With
tri-n-propylamine;
In
1-methyl-pyrrolidin-2-one;
at 175 ℃;
for 24h;
Inert atmosphere;
|
34% |
|
With
dimethylaminoacetic acid;
bis(benzonitrile)palladium(II) dichloride;
|

1-bromo-4-methoxy-benzene

2-Ethylhexyl acrylate
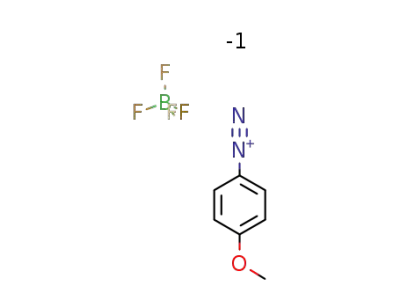
4-methoxybenzenediazonium tetrafluoroborate
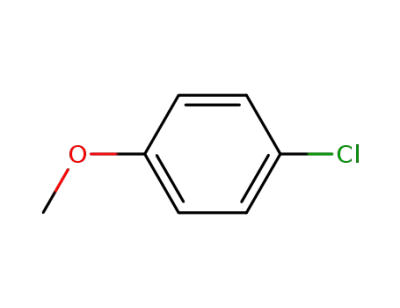
4-chloromethoxybenzene
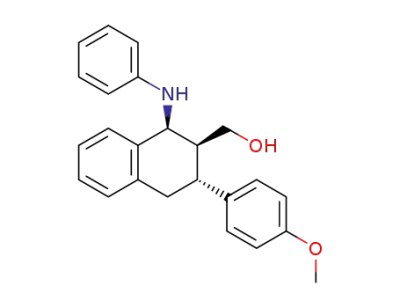
2-hydroxymethyl-3-(p-methoxyphenyl)-1-phenylamino-1,2,3,4-tetrahydronaphthalene

C24H23NO2
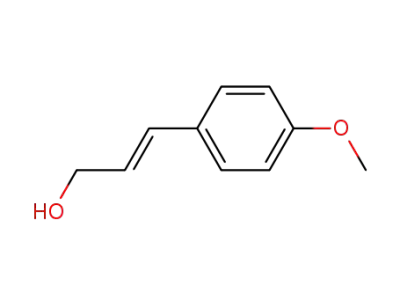
(E)-p-methoxy-cinnamyl alcohol
4-methoxy-trans-cinnamaldehyde
CAS:138071-82-6
CAS:57280-22-5
CAS:105-56-6
CAS:92761-26-7